- Natural Sciences and Science Education, National Institute of Education, Nanyang Technological University, Singapore, Singapore
Plants are known to have many secondary metabolites and phytochemical compounds which are highly explored at biochemical and molecular genetics level and exploited enormously in the human health care sector. However, there are other less explored small molecular weight proteins, which inhibit proteases/proteinases. Plants are good sources of protease inhibitors (PIs) which protect them against diseases, insects, pests, and herbivores. In the past, proteinaceous PIs were considered primarily as protein-degrading enzymes. Nevertheless, this view has significantly changed and PIs are now treated as very important signaling molecules in many biological activities such as inflammation, apoptosis, blood clotting and hormone processing. In recent years, PIs have been examined extensively as therapeutic agents, primarily to deal with various human cancers. Interestingly, many plant-based PIs are also found to be effective against cardiovascular diseases, osteoporosis, inflammatory diseases and neurological disorders. Several plant PIs are under further evaluation in in vitro clinical trials. Among all types of PIs, Bowman-Birk inhibitors (BBI) have been studied extensively in the treatment of many diseases, especially in the field of cancer prevention. So far, crops such as beans, potatoes, barley, squash, millet, wheat, buckwheat, groundnut, chickpea, pigeonpea, corn, and pineapple have been identified as good sources of PIs. The PI content of such foods has a significant influence on human health disorders, particularly in the regions where people mostly depend on these kind of foods. These natural PIs vary in concentration, protease specificity, heat stability, and sometimes several PIs may be present in the same species or tissue. However, it is important to carry out individual studies to identify the potential effects of each PI on human health. PIs in plants make them incredible sources to determine novel PIs with specific pharmacological and therapeutic effects due to their peculiarity and superabundance.
Introduction
Protease inhibitors (PIs) are well known as one of the prime candidates to have numerous applications in biotechnology and medicine. These are very important tools for better interpretation of basic principles of protein interactions and the designing of new compounds for the control of pathologic processes and many diseases (Lingaraju and Gowda, 2008). More than half of the world's population still depends completely on plants and their medicinal products. Until now, plants have served as starting raw materials for numerous drugs on the market. From ancient time, plant extracts containing proteolytic enzymes have been used in traditional medicine (De Feo, 1992). In Ayurvedic, Homeopathy, Unani and even in Allopathic systems, medical practitioners, and producers suggest and use several medicines that are prepared from whole plants or plant parts or phytochemicals such as secondary metabolites. Unlike primary and secondary metabolites which comprise of an array of several thousands of compounds, there are other compounds which are still less explored among plant natural products, particularly the proteins of low molecular weight. Protease inhibitors are among the most important groups of such proteins. Similar to animals and micro organisms, plants also contain several different types of protease inhibitors.
It has been reported that the occurrence rates of breast, colon, and prostate cancer are low in a population consuming a higher amount of seeds like beans, maize and rice (Correa, 1981). There are many lines of evidence from in vitro, in vivo, epidemiological and clinical trial data that demonstrate a plant-based diet decreasing the risk of chronic diseases, cancer in particular (Hasler, 1998). Block (1992) reported a review of 200 epidemiological studies which indicated that the cancer possibility in people who ate more fruits and vegetables was only one-half of that in those who ate a few of these foods. Thus, good nutrition is a recognized key to disease prevention. Also, health professionals are gradually recognizing the role of phytochemicals in disease prevention and health promotion. Recently, the molecular basis of disease prevention by nutritional involvement has been under scientific consideration whereby in the past, food intake has shown the ability to influence the initiation or progression of chronic diseases (Dirsch and Vollmar, 2001; Losso, 2008). Nowadays dietary factors have also attracted attention as cancer prevention agents due to their pharmacological safety. The abundance of plants can be used either as raw materials “as it is” or processed further to generate concentrated bioactive components with therapeutic action like flavonoids and phenolic acids that comprise powerful anti-oxidant activities (Pietta, 2000; Lizcano et al., 2010).
Food intake shows a strong influence on health (Losso, 2008). Instead of pills, functional foods can be consumed as part of a normal diet. Hence, plant protease inhibitors (PPIs) fit the definition of a functional food. Many researchers have classified these plant protease inhibitors into families such as Bowman-Birk, Kunitz, Potato I, Potato II, Serpine, Cereal, Rapeseed, Mustard, and Squash (Laskowski and Qasim, 2000; De Leo et al., 2002). Naturally occurring PIs are abundant in legume seeds. Serine protease Bowman-Birk inhibitors (BBI) and Kunitz-type inhibitors (KTI) have been studied extensively as compared to the other in these legumes (Ryan, 1990). These families differ in their mass, cysteine content, and the number of reactive sites (Lingaraju and Gowda, 2008). BBIs, which molecular weights range from 8000–10000 Da, are double-headed serine protease inhibitors with 71 amino acids and 7 disulphide bonds (Odani and Ikenaka, 1973). Each BBI has two functional active sites on opposite sides of the molecule, which inhibits both trypsin and chymotrypsin-like proteases (Clemente et al., 2011). Whereas KTIs are 8000–22,000 Da proteins with two disulphide linkages and a single reactive site of trypsin (Laskowski and Qasim, 2000). Among all, BBI is identified as a suitable protein to work with, in terms of handling, resistance to temperature extremities and acidification (Fields et al., 2012). Naturally occurring plant based BBI is considered as a therapeutically significant candidate in the treatment of multiple diseases, especially in the field of cancer prevention (Fields et al., 2012). Perhaps its greatest potential in therapeutic property lies in its ability to suppress carcinogenesis irreversibly even during early stage (Wattenberg, 1992). Encouragingly, the amount of BBI that reaches the internal organs after oral ingestion reaches the range that prevents malignant transformation in vitro (Yavelow et al., 1985; Fields et al., 2012). Several PPIs are under further evaluation in human clinical trials.
Protease inhibitors designed for therapeutic applications are quickly advancing due to the ever extending establishment of key information provided by the protein chemists and enzymologists working in this field. In this review, we focus on the role of plant proteases and their inhibitors in human diseases, and on the possible application of proteinaceous plant PIs as drugs. We also will discuss the several criteria to be met before such drugs are applicable to clinical trials.
Roles of Plant Protease Inhibitors in Health and Disease Control
The widespread distribution of protease inhibitors throughout the plant kingdom is well known since 1938 (Ryan, 1973). In general, these PIs comprises about 5–10% of the total content of water-soluble proteins found in the seeds of dicots and monocots of angiosperms and in gymnosperms (Mutlu and Gal, 1999). However, the most well-studied protease inhibitors of plant origin are from three main families namely, Fabaceae, Poaceae, and Solanaceae (Richardson, 1991). Weder (1981) reported that the seed protein of the legumes enriched with up to 6% of PIs, whereas cereal contains about 10% of PIs (Pusztai, 1972). Later, many studies have reported PIs found in other families such as Malvaceae, Rutaceae, Poaceae and Moringaceae (Bijina et al., 2011). These natural PIs mainly accumulate in tubers, seeds, and leaves.
Medicinal plant biotechnology has emerged as a revolutionary methodology which is useful to induce the formation and accumulation of desirable compounds and eventually develop the therapeutic product (Constabel, 1990). Therefore, it is indispensable to select locally available edible plant species or plant extracts that could practically be added to the available drugs list, or even replace some expensive compounds that need to be utilized in pharmaceutical preparations. The investigation to search for PIs to combat several clinical disorders started in early 1950's (Vogel et al., 1968). For many years, several researchers have isolated and purified these plant PIs from different plant species and examined them as therapeutic agents using in vitro methods. Many of those naturally found PIs were further characterized from different plant species which mainly included trypsin from serine protease group which have been tested for various diseases (Richardson, 1991; Tamir et al., 1996; Majumdar, 2013). This review explains about PIs of all earlier reported plant species that have been used as therapeutic agents and tested against different diseases and human disorders (Table 1; Murugesan et al., 2001; Neuhof et al., 2003; Troncoso et al., 2003; Kobayashi et al., 2004; Lanza et al., 2004; Clemente et al., 2005, 2012; Kim et al., 2005; Suzuki et al., 2005; Capaldi et al., 2007; Banerjee et al., 2008; Tochi et al., 2008; Caccialupi et al., 2010; Hsieh et al., 2010; Joanitti et al., 2010; García-Gasca et al., 2012; Magee et al., 2012; de Paula et al., 2012a; Borodin et al., 2013; Ferreira et al., 2013; Rakashanda et al., 2013b; Clemente and Arques, 2014; Souza et al., 2014) and future scope to search for new species, which are as follows:
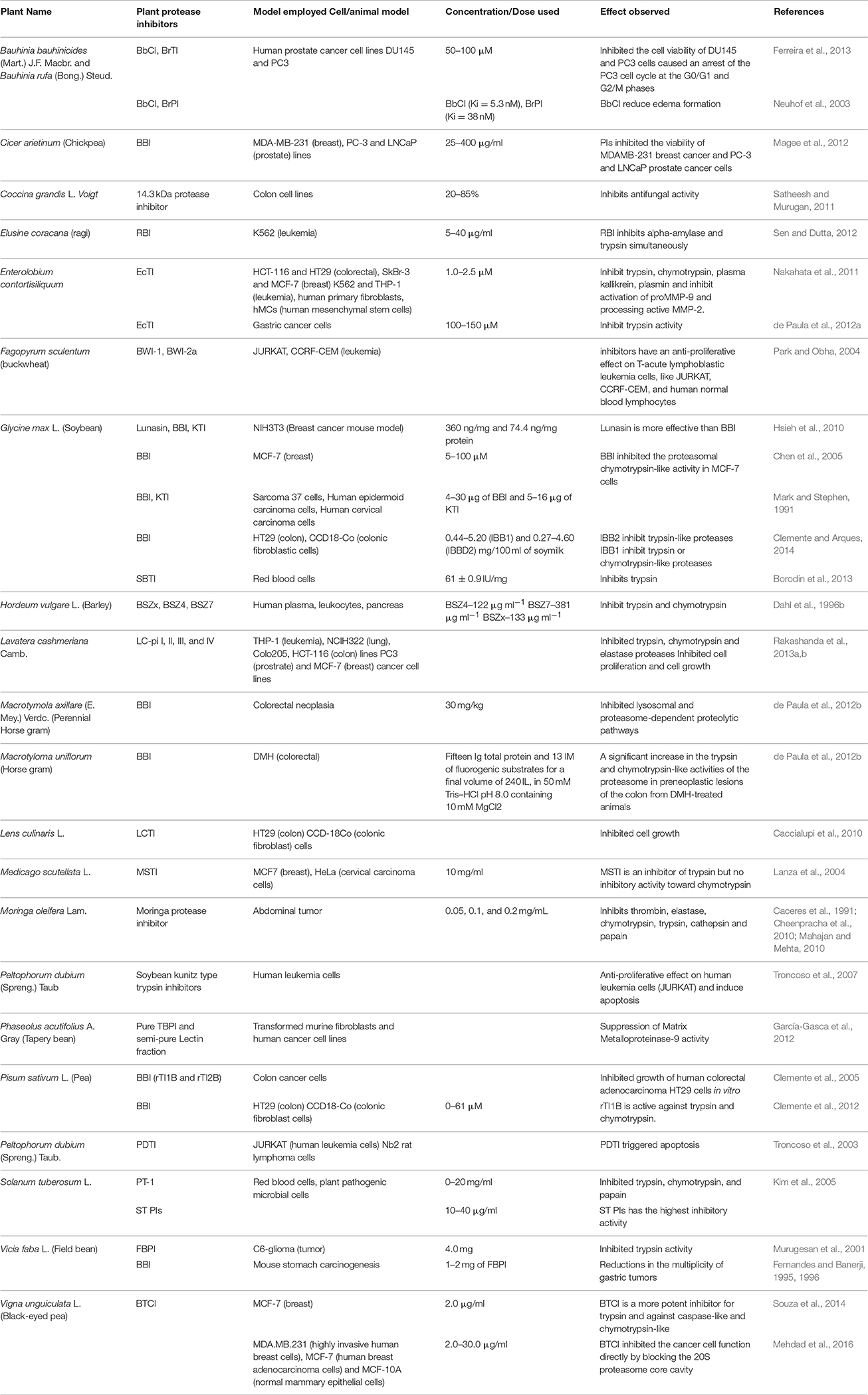
Table 1. Pre-clinical (in vitro) studies and pharmacological effects of plant protease inhibitors (PPIs) in disease prevention.
Ananas comosus (L.) Merr. (Pineapple)
Bromelain proteases from pineapple extracts (Fruit bromelain) are clinically used as anti-inflammatory agents (Ammon, 2002; Darshan and Doreswamy, 2004; Lemay et al., 2004) for colonic inflammation, chronic pain, rheumatoid arthritis, soft tissue injuries, and asthma (Izaka et al., 1972; Cooreman et al., 1976; Taussig and Batkin, 1988; Kelly, 1996; Maurer, 2001; Jaber, 2002; Hale et al., 2005). Similar folding and disulfide bond connectivity were found to be shared in Bromelain inhibitor VI from pineapple stem (Stem bromelain, BI-VI) with the Bowman-Birk trypsin/chymotrypsin inhibitor from soybean (BBI-I) (Hatano et al., 1996). It is essentially a group of sulfhydryl proteolytic enzymes that includes a variety of cysteine proteases (Tochi et al., 2008). Along with protease inhibitors, it also encompasses peroxidases, acid phosphatase, organically bound calcium and remains stable over a wide range of pH 2–9 (Hatano et al., 1996; Tochi et al., 2008). Even though, both stem bromelain and fruit bromelain are single-chain glycosylated enzymes, stem bromelain has low proteolytic ability and specificity for peptide bonds compared to fruit bromelain. Similarly, the molecular weight ranges for stem bromelain and fruit bromelain are 26–37 kDa and 24.5–32 kDa respectively (Grzonka et al., 2007; Gautam et al., 2010; Kumar et al., 2011).
It is reported that bromelain with its proteolytic nature is well absorbed orally in a dose-dependent manner (Hatano et al., 1996). Proteolytic activity of bromelain has been shown to play a minor part in pharmacological activity while other factors of it, such as immune-modulatory, hormone-like properties, fibrinolytic activity and uncharacterized components such as CCS and CCZ compliments also contributes toward its pharmacological activity (Tochi et al., 2008). However, it is necessary to investigate further on these uncharacterized components. Investigating how the uncharacterized components can be incorporated into daily food is important to determine if it is safe and non-toxic (Tochi et al., 2008).
Bauhinia bauhinioides (Mart.) J.F. Macbr. and Bauhinia rufa (Bong.) Steud.
Bauhinia seeds are rich in serine and cysteine proteases inhibitors (Oliva and Sampaio, 2008; Oliva et al., 2010, 2011). Oliva et al. (1999) isolated the Bauhinia bauhinioides kallikrein inhibitor (BbKI) from seeds of B. bauhinioides, which is a 18-kDa protein with a similar primary structure to that of other plant Kunitz-type inhibitors but is devoid of disulphide bridges. BbKI was able to inhibit plasma kallikrein, plasmin, bovine trypsin, bovine chymotrypsin, porcine pancreatic kallikrein, and murine plasma kallikrein (Oliva et al., 1999). Nakahata et al. (2006) isolated another inhibitor from Bauhinia rufa and named it as BrTI (B. rufa trypsin inhibitor) that can inhibit human plasma kallikrein (Ki app = 14 nM) in addition to trypsin (Ki app = 2.9 nM).
Kunitz-type protease inhibitor from B. bauhinioides and B. rufa are reported to reduce the edema formation in isolated perfused rabbit lung (Neuhof et al., 2003). Kunitz-type Inhibitor BbCI (10(-5) M) from B. bauhinioides significantly decreased the pulmonary edema in isolated perfused rabbit lungs caused by activated neutrophils via release of elastase to the same degree as by eglin C (10(-5) M) from Hirudo medicinalis, which was used as a reference (Neuhof et al., 2003). Kallikreins play an important role in the establishment of a common prostate cancer. RGD/RGE motifs of the inhibitor BrTI from Bauhinia rufa is included into rBbKIm to form the recombinant B. bauhinioides kallikreins (Ferreira et al., 2013). This rBbKIm inhibited trypsin (Ki app = 1.6 nM), chymotrypsin (Ki app = 7.4 nM), and human plasma kallikrein (Ki app = 3.6 nM). Also, it was reported that the viability of fibroblasts was not affected when rBbKIm inhibited the cell viability of DU145 and PC3 prostate cancer cells (Ferreira et al., 2013).
Cicer arietinum L. (Chickpea)
Protease inhibitor concentrates extracted from Chickpea seeds which enriched in BBI-type PIs exhibited inhibitory activity against chymotrypsin (Magee et al., 2012). Viability of MDAMB-231 breast cancer and PC-3 and LNCaP prostate cancer cells were inhibited significantly by Chickpea Bowman-Birk-type protease inhibitor (molecular weight ~8 kDa) at all concentrations tested (25–400 μg/ml; Magee et al., 2012). The study also suggested that chickpea PIs may contain same anticancer properties like soybean BBI and hence deserves further study as a potential chemopreventive agent (Magee et al., 2012).
Coccinia grandis (L.) Voigt.
The Coccinia grandis protease inhibitors (CGPI) is a protein of 14.3 kDa isolated from its leaves. CGPI showed a highest inhibitory activity against both bovine pancreatic trypsin and chymotrypsin. The antimicrobial activity of thses CGPIs has been reported by Satheesh and Murugan (2011). CGPIs exhibited significant growth inhibitory effect on colon cell lines via dose-dependent manner. These CGPIs also inhibited mycelial growth and sporulation of pathogenic microbial strains such as Staphylococcus aureus, Klebsiella pneumonia, Escherichia coli, Proteus vulgaris, Bacillus subtilis and pathogenic fungus Mucorindicus, Candida albicans, Pencilliumnotatum, Aspergillus flavus, and Cryptococcus neoformans (Satheesh and Murugan, 2011). PIs-treated fungus showed a significant shrinkage of hyphal tips. These results indicate that the PIs extracted from C. grandis will be an excellent compound to develop oral or other anti-infective agents.
Elusine coracana Gaertn. (ragi)
Generally in many regions, finger millet or ragi is considered as a staple food crop for its excellent nutritional qualities, long storage capability, and medicinal properties. Sen and Dutta (2012) reported that purified ragi (Elusine coracana) is a 14 kDa bifunctional inhibitor (RBI), a member of cereal trypsin/α-amylase inhibitor family that simultaneously inhibits α-amylase and trypsin forming a ternary complex (Maskos et al., 1996). Its primary structure is a monomeric protein of 122 amino acids containing five intramolecular disulfide bonds (Campos and Richardson, 1983). The RBI reduced cellular proliferation and induced apoptosis of chronic myeloid leukemia cell, K562. These purified RBI exhibited cytotoxicity toward K562 chronic myeloid leukemia cells, but not against normal human peripheral blood mononuclear cells. This investigation may provide a future preventive as well as a natural therapeutic solution for chronic myeloid leukemia.
Enterolobium contortisiliquum (Vell.) Morong
It is a flowering tree in the pea family, Fabaceae. The purified Enterolobium contortisiliquum Trypsin Inhibitor (EcTI) is a 20 kDa protein of a Kunitz-type inhibitor from the seeds. EcTI inhibited the activity of trypsin, chymotrypsin, plasma kallikrein, and plasmin (Nakahata et al., 2011). The effects of a EcTI on adhesion, migration, and invasion of gastric cancer cells were reported by de Paula et al. (2012a). EcTI was shown to reduce the expression without affecting the fibroblasts upon adhesion and interrupt the cellular organization of molecules such as integrin β1, cortactin, MT1-MMP, MMP-2, and N-WASP, which are involved in the establishment and maturation of invadopodia. EcTI decreased Src-FAK signaling thereby inhibited the adhesion, migration and cell invasion (de Paula et al., 2012b). It was clear from the study that EcTI inhibited the invasion of gastric cancer cells by manipulating the integrin-dependent cell signaling pathways. Furthermore, Nakahata et al. (2011) reported the inhibition of human cancer cell lines, such as HCT116 and HT29 (colorectal), K562 and THP-1 (leukemia), SkBr-3 and MCF-7 (breast), as well as on human primary fibroblasts and human mesenchymal stem cells (hMSCs) upon treatment with EcTIs. Data indicates that the EcTI is an important tool in the studies of tumor cell development and distribution as it prevents proMMP activation, and is cytotoxic against tumor cells without disturbing normal tissue.
Fagopyrum sculentum Moench (Buckwheat)
A buckwheat inhibitor (BWI)-1 protein extracted from common buckwheat seeds with a molecular weight of 7.7 kDa (Dunaevsky et al., 1997). BWI-1 is from the potato inhibitor I family which can inhibit trypsin, chymotrypsin, and subtilisin, whereas BWI-2a is a new PI, homologous to the vicilin family that can only inhibit trypsin (Lim, 2013). The inhibitory activity of BWI-1 and BWI-2a against T-acute lymphoblastic leukemia (T-ALL) cells, such as JURKAT and CCRF-CEM and human normal blood lymphocytes has been reported previously (Park and Obha, 2004; Lim, 2013). These two inhibitors induce apoptosis in these cells with DNA fragmentation.
Glycine max (L.) Merr. (Soybean)
The seeds of Glycine max possess both BBI and KTI. Its BBI is an 8 kDa protein and is able to reduce the proteolytic activities of trypsin, chymotrypsin, elastase, cathepsin G, and chymase, serine protease-dependent matrix metalloproteinases, urokinase protein activator, mitogen activated protein kinase, and PI3 kinase, and upregulates connexin 43 (Cx43) (Losso, 2008). Whereas the molecular weight of KTI is approximately 22 kDa. A concentrated protein extract of soybean, known as Bowman-Birk inhibitors concentrate (BBIC) which is enriched with BBI, is a present investigational new drug. The name of the family Bowman-Birk inhibitors is coined after D. E. Bowman and Y. Birk, who identified and characterized the typical member of this family, the soybean inhibitor from soybean (Glycine max; Bowman, 1946; Birk, 1961). In rodents, soybean BBI treatment has a potent suppressive effect on colon and anal gland inflammation, following exposure to carcinogenic agents (Billings et al., 1990). Soybean BBI reduced the initiation and regularity of colorectal tumors at low concentrations of 10 mg/100 g diet in the dimethylhydrazine (DMH) rat model, without any adverse effect on the animal growth or organ physiology (Kennedy et al., 2002). This suppressive effect on the colorectal tumor development was disappeared upon the reduction of BBI inhibitory activity, indicating that the BBI inhibitory activity against serine proteases may be necessary for the chemopreventive properties (Figure 1). The protease activities, which plays a major role in tumorigenesis, are deregulated in colorectal cancer and neoplastic polyps (Chan et al., 2010).
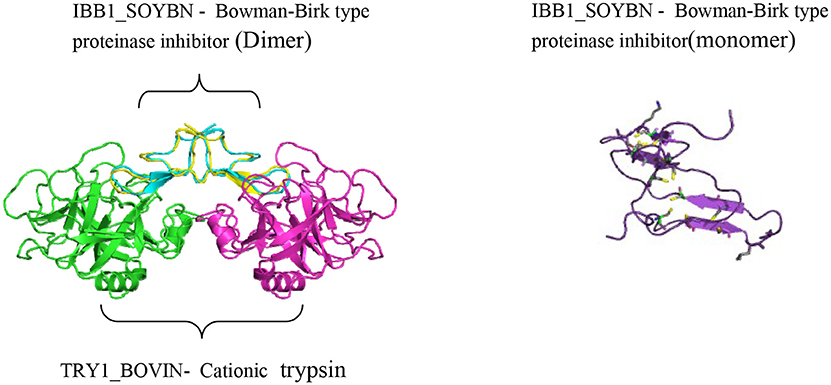
Figure 1. Crystal structure of cancer chemopreventive Bowman-Birk inhibitor from Soybean in a ternary complex with bovine trypsin (PDB id: 1d16r) (Koepke et al., 2000).
Ware et al. (1999) reported that soybean BBI inhibited the proteases of inflammation-mediating cells and suppressed the superoxide anion radical production from the immunocytes. This study also indicated that BBIC can positively influence the dextran sulfate sodium-treated mice, which in turn useful in treating the human inflammatory bowel diseases, mainly ulcerative colitis. Another study reported that BBI is an anti-carcinogenic serine protease inhibitor that may obstruct the protease activity of prostate-specific antigen (PSA) and the development of human prostate cancer xenografts in nude mice (Wan et al., 1999b).
The role of soybean BBI in suppressing the development of activated oxygen species from prostate cancer cells (Sun et al., 2001), and in triggering DNA repair via a p53-dependent mechanism have been reported (Kennedy et al., 2002). In the recent studies, BBIC has prevented the growth of prostate tumors by its antiproliferative activity through stimulation of connexin 43 expressions in transgenic rats (McCormick et al., 2007; Tang et al., 2009).
The suppressive activity of soybean BBI, on HT29 colon cancer cells, compared with CCD-18Co cells of non-malignant colonic fibroblast was studied by Clemente et al. (2010). The study indicated that the BBI treatment in a dose-dependent manner, blocked the G0-G1 phase to disrupt the cell cycle distribution pattern of HT29 colon cancer cells. Chen et al. (2005) reported the BBI as a potential inhibitor which inactivated the MCF7 cancer cells both in vitro and in vivo by inhibiting 26S proteasomal chymotrypsin-like activity. The study also indicated that the BBI arrested the growth of MCF7 cells at G1/S phase, by accumulating MKP-1 causing interruption in the ubiquitin-proteasome pathway which resulted in suppressed ERK1/2 activity. Being a potential chemotherapeutic drug, BBI obstructs the cell proliferation and viability at different stages of cancer development. Hence, the BBI involving proteasome inhibition is considered as a new mechanism which may be a potential reason for its chemopreventive properties. Eventually, the study on soybean BBI proteins has provided an information on the therapeutic interference of soybean BBI for breast cancer treatment.
Kobayashi et al. (2004) studied the effects of soybean trypsin inhibitor (SBTI) on the enzymatic activity of extracellular uPA and signal transduction mechanisms that are involved in its expression and incursion into HRA human ovarian cancer cells. Authors concluded that KTI could stop cell invasiveness by reduction of uPA signaling instead of BBI although the mechanisms of KTI may not be the same as those of Bikunin. BBI inhibited M5067 ovarian sarcoma by increasing expression of tumor-suppressor molecules Cx43 (Suzuki et al., 2005).
Oral administration has been recognized as a possible and cost-effective approach to reducing cancer morbidity and mortality by inhibiting precancerous events before the occurrence of clinical disease (Prasain and Barnes, 2007). Hsieh et al. (2010) found that lunasin is actually the bioactive cancer preventive agent in BBIC and BBI simply protects lunasin from digestion when soybean and other seed food are eaten by a human. Currently, the rising of breast cancer cases urged to identify novel compounds that can be utilized as preventive or therapeutic agents. Evidence is shown from animal experiments, epidemiological studies, and human surveys of people consuming a soy-rich diet exhibited lower disease occurrence and mortality from breast cancer which leads to more research on various compounds from soy that can prevent breast cancer (Adlercreutz et al., 1995; Banerjee et al., 2008; Hernández-Ledesma et al., 2009). Consuming such anticancer compounds daily could be an alternative to chemotherapy, which is safe to the physiology of normal tissue and prevents initiation of micro tumors (Béliveau and Gingras, 2007; de Kok et al., 2008). Furthermore, it is important to assess the possible risks and advantages of phytochemicals to human health by understanding the physiological behavior of these compounds after oral intake which includes absorption, distribution, metabolism and excretion (Prasain and Barnes, 2007).
Borodin et al. (2013) reported that the effect of both proteins, soybean SBTI and aprotinin, on coagulation and thrombocyte hemostasis by in silico and in vitro methods and demonstrated the inhibition of blood clotting, fibrinolysis and platelet aggregation (Figure 5). These outcomes were accomplished by increased prothrombin time, activated partial thromboplastin time, activated clotting time, thrombin time as well as prevention of fibrinolysis.
Hordeum vulgare L. (Barley)
In 1970's, a group of ~43 kDa proteins from mature grains of barley (Hordeum vulgare L.) were identified as members of the serpin superfamily (Hejgaard et al., 1985). There are three mostly recognized barley serpin subfamilies: BSZ4, BSZ7, and BSZx. Barley serpins are effective, irreversible inhibitors of serine proteases of the chymotrypsin family. The Barley serpin, BSZx inhibits both chymotrypsin and trypsin at overlapping reactive sites (Dahl et al., 1996a). BSZx serpin interacts with a range of serine proteases from human plasma, leukocytes, pancreas, a fungal trypsin and three subtilisins (Dahl et al., 1996b). The study indicated that BSZx inhibitors which inhibited trypsin and chymotrypsin are also capable to inhibit coagulation factors such as thrombin, plasma kallikrein, Factor VIIa and Factor Xa.
Ipomoea batatas L. (Sweet Potato)
The anti-proliferative effect and the mechanism of a 22 kDa trypsin inhibitor (TI) protein from sweet potato (Ipomoea batatas (L.)) storage roots on NB4 promyelocytic leukemia cells was reported by Huang et al. (2007). TI arrested cell cycle at the G1 phase as determined by flow cytometry analysis and apoptosis as shown by DNA laddering. TI-induced cell apoptosis involved p53, Bcl-2, Bax, and cytochrome c protein in NB4 cells. P53 and Bax proteins accumulated, and antiapoptotic molecule Bcl-2 decreased in the tested cells in a time-dependent manner during TI treatment. The study indicated that TI stimulates the apoptosis of NB4 cells by blocking the cell growth and activating the pathways of caspase-3 and -8 cascades.
Lavatera cashmeriana Camb.
It belongs to the Malvaceae family, which is indigenous to Kashmir valley, has incredible medicinal values. For many years, its plant parts are being utilized to cure cold and sore throat (Rakashanda et al., 2013a). Lavatera cashmeriana protease inhibitors (LC-pi I, II, III, and IV) were extracted from its seeds and are considered as a Kunitz type of inhibitor based on their molecular size (Rakashanda et al., 2012, 2013a). These protease inhibitors were able to inhibit trypsin, chymotrypsin, and elastase. Four serine protease inhibitors appeared as 20.9, 14.1, 16.8, and 7.9 kDa peaks in gel filtration and 10, 14, 16, and 7 kDa bands by SDS PAGE. Representing that LC-pi I includes two similar subunits of 10 kDa. LC-pi I tested against Klebsiella pneumoniae and Pseudomonas aeruginosa which showed strong antibacterial activity but was less active against Escherichia coli (Figure 5). However, all four inhibitors demonstrated the in vitro anticancer activity on THP-1 (leukemia), NCIH322 (lung), and Colo205, HCT-116 (colon). Among all, LC-pi I and II were treated as potential anticancer agents (Rakashanda et al., 2013b). Moreover, a strong inhibitory effect of LC-pi I was exhibited in in vitro conditions on the initiation of the prostate (PC-3) and breast (MCF-7) cancer cells lines because of its protease inhibitor activity of trypsin, chymotrypsin, and elastase (Rakashanda et al., 2013a).
Lens culinaris L. (Lentil)
Ragg et al. (2006) reported the isolation of a lentil (Lens culinaris, L., var. Macrosperma) seed trypsin inhibitor (LCTI) and its functional and structural characterization. LCTI is a 7.4 kDa double-headed trypsin/chymotrypsin inhibitor (Figure 2). Further, Caccialupi et al. (2010) isolated a full-length cDNA, encoding a BBI from lentil seeds. The effects of this mature BBI on the growth of human colon adenocarcinoma HT29 and colonic fibroblast CCD-18Co cells were evaluated. LCTI was able to inhibit the growth of such cells at concentrations as low as 19 μmol/L, in a concentration-dependent manner without affecting the CCD-18Co cells.
Macrotymola axillare (E. Mey.) Verdc. (Perennial Horse Gram)
Joubert et al. (1979) isolated and characterized two protease inhibitors, DE-3 and DE-4 from Macrotyloma axillare seeds. The amino acid sequences of two protease inhibitors were compared with the known sequences of the BBIs which resulted in 67% homology and corresponding properties of the BBI double-headed protease inhibitor group. Each of them comprise 76 amino acid residues including 14 half-cystine residues. BBI from the M. axillare worked against inflammation and initiation of pre-neoplastic lesions in the induced DMH mouse model as reported by de Paula et al. (2012b). In this study, the potential ability of BBI preparations was examined for the prevention of colorectal neoplasia caused by intraperitoneal injections of 1,2-dimethylhydrazine (DMH) by using 30 mg/kg dosage over a period of 12 weeks. Histopathological variations consistent with tumor development, increased CD44 expression and proteasome peptidase activities were exhibited by the DMH-treated mice. It is demonstrated that the BBI inhibited both lysosomal and proteasome-dependent proteolytic pathways through which it could prevent the development of pre-neoplastic lesions.
Medicago scutellata L. (Snail Medic)
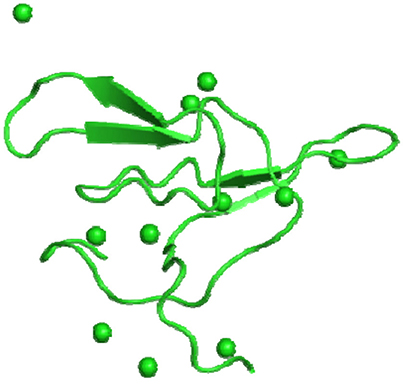
Medicago scutellata L. (Snail medic) seeds stores a significantly higher content of a trypsin inhibitor (MsTI) compared to Medicago species. MsTI belongs to the BBI family of serine proteases and exhibited a highest sequence homology with the soybean BBI (Catalano et al., 2003). It consists of 62 residues corresponding to a molecular mass of 6.9 kDa. Catalano et al. (2003) reported the anticarcinogenic BBI, which is a high-resolution three-dimensional structure purified from snail medic seeds (Medicago scutellata) (MSTI) (Figure 3). Later, Capaldi et al. (2007) reported the ternary complex structure of the BBI that is purified from snail medic seeds (MSTI) and two molecules of bovine trypsin. The effects of MsTl on cell killing induced by cisplatin in MCF7 human breast carcinoma cells and HeLa cervical carcinoma cells were evaluated by Lanza et al. (2004). After 24 h of MsTI treatment with cell culture medium, resulted in increased cisplatin-induced cytotoxicity and reduced the clonogenic survival of MCF7 and HeLa cells in a dose-dependent manner. In comparison with the similar ternary complex of the SBTI, this model shows very little differences in the polypeptide chain of the trypsin binding sites. The area between Asp 26 and His 32 of the MSTI has the largest difference whereby the soybean inhibitor has an extra Leu inserted in position 29 (Catalano et al., 2007).
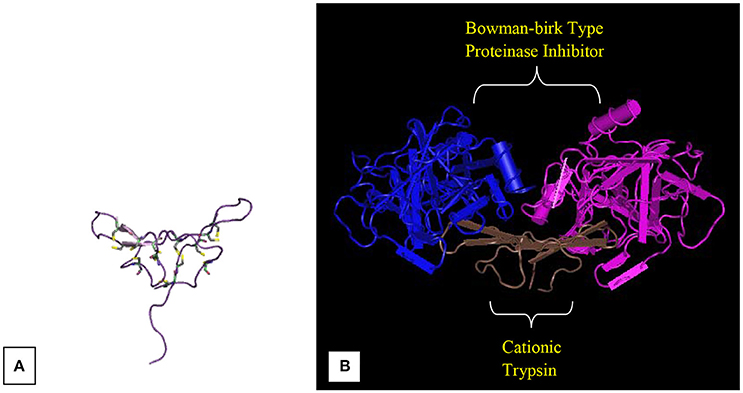
Figure 3. Bowman-Birk inhibitors of Medicago scutellata. (A) IBB_MEDSC-Bowman-Birk type protease inhibitor (PDB id: 1mvz). (B) The anticarcinogenic Bowman-Birk inhibitor from snail medic (Medicago scutellata) seeds complexed with bovine trypsin (Capaldi et al., 2007).
Moringa oleifera Lam.
It belonging to the family Moringaceae, recorded high level of protease inhibitor activity (92%) against trypsin (Bijina et al., 2011). PI extracted from M. oleifera is a small protein with a molecular weight of 23.6 kDa. Its molecular mass and and the disulphide content of the polypeptide indicated that Moringa PI belongs to the Kunitz type of serine protease inhibitor family (Bijina et al., 2011). M. oleifera grows well throughout the tropics and almost every part of the plant is of value as food. The flowers, leaves, and roots are used in folk remedies for the treatment of tumors and the seeds for abdominal tumors. The bark is regarded as antiascorbic and exudes a reddish gum with properties of tragacanth is sometimes used for diarrhea. Roots are bitter and act as a tonic to the body and lungs. They are used as an expectorant, mild diuretic, stimulant in paralytic afflictions, in epilepsy, and in hysteria (Hartwell, 1971). Moreover, there are a few low molecular weight bioactive compounds present in Moringa seeds which exhibited bactericidal, fungicidal, immune suppressive activities (Mahajan and Mehta, 2010) and some anti-inflammatory agents (Caceres et al., 1991; Cheenpracha et al., 2010). Moringa PI is the potential source for the development of a new drug against thrombin, elastase, chymotrypsin, trypsin, cathepsin, and papain in pharmaceutical industries (Bijina et al., 2011). Furthermore, these PIs could also be used as a seafood preservative against proteolysis and in other biotechnological applications.
Phaseolus acutifolius A. Gray (Tepary Bean) and Phaseolus vulgaris L.
Bowman-Birk PI from Tepary bean (TBPI) seeds with anti-trypsin and antichymotrypsin activities was isolated and characterized by Campos et al. (1997). García-Gasca et al. (2012) again purified and characterized 7 kDa TBPI as described by Campos et al. (1997). However, this purified TBPI did not show cytotoxicity but it was responsible for the increase in cell adhesion and decrease in extracellular matrix degradation in cell culture which leads to a decreased in vitro cell invasion capacity and suppression of matrix metalloproteinases-9 activity simultaneously. According to this study, the Tepary bean seeds contain minimum two separate groups of bioactive proteins with anticancer properties (García-Gasca et al., 2012). The study also reported that cells treated with TBPI diminished their invasive ability most probably due to the suppression of MMP2 and MMP9. Sun et al. (2010) isolated and purified two trypsin inhibitors of each with a molecular mass of 16 kDa from Phaseolus vulgaris cv “White Cloud Bean.” Both of these inhibitors showed an antiproliferative activity toward Leukemia L1210 albeit with a little variance in potency, but there was little activity toward lymphoma MBL2 cells.
Pisum sativum L. (Pea)
The crystal structure of the Pisum sativum (PsTI) isoform was determined by molecular replacement at 2.7A resolution using the X-ray coordinates of the soybean inhibitor as a search model (Li de la Sierra et al., 1999). Protease inhibitors, rTI1B, and rTI2B from P. sativum L. (Pea) are homologous to BBI with molecular mass range 7–9 kDa, but differ in inhibitory activity, on the growth of human colorectal adenocarcinoma HT29 cells in vitro (Clemente et al., 2005, 2012). The rTI1B proved to be active against trypsin and chymotrypsin, whereas the related mutant protein was inactive against both serine proteases. The proliferation of HT29 colon cancer cells was notably affected by rTI1B in a dose-dependent manner, whereas the inactive mutant did not show any significant activity on cell growth of colon cancer. Likewise, none of the recombinant proteins affected the growth of non-malignant colonic fibroblast CCD-18Co cells. From the findings, it was clear that serine proteases are important candidates in investigating the potential cancer preventive trait of BBI in the initial stages of colorectal cancer (Clemente et al., 2012; Clemente and Arques, 2014).
Peltophorum dubium (Spreng.) Taub.
Troncoso et al. (2003) isolated a trypsin inhibitor, PDTI of molecular mass range 20–22 kDa from Peltophorum dubium seeds. The amino-terminal sequences of PDTI were the same as Kunitz-type soybean trypsin inhibitor (SBTI). Both PDTI and SBTI triggered apoptosis of Nb2 rat lymphoma cells via reducing viability, DNA fragmentation, DNA hypodiploidy and caspase-3-like activity. However, they did not damage normal mouse splenocytes or lymphocytes but caused apoptosis of concanvalin A-stimulated mouse lymphocytes (Troncoso et al., 2003). Many findings reported about the anti-proliferative effect and apoptosis in human leukemia cells (JURKAT) via PDTI and SBTI activity. In addition, human peripheral lymphocytes, stimulated with phytohemagglutinin or not, are also sensitive to viability decrease that is induced by SBTI (Troncoso et al., 2007).
Pseudostellaria heterophylla Rupr. & Maxim. (Ginseng)
Wang and Ng (2006) isolated a 20.5 kDa trypsin inhibitor of Kunitz-type with antifungal activity (Figure 5) and a new lectin from roots of P. heterophylla. They exhibited a trypsin inhibitory activity that is similar to soybean trypsin inhibitor. Antifungal activity was also demonstrated toward Fusarium oxysporum like sprotinin and Kunitz-type trypsin inhibitors from soybeans and lima beans.
Solanum tuberosum L.
Although protease inhibitors are found in plants belonging to different kinds of systematic groups, high levels of protease inhibitors are often reported in many plants belonging to the Solanaceae family (Plate et al., 1993). The effect of a protease inhibitor extracted from potatoes (POT II) which increase CCK release, on food intake was examined in 11 lean subjects by Hill et al. (1990). The findings suggested that endogenous CCK may be important in the control of food intake and that protease inhibition may have therapeutic potential for reducing food intake. Several antimicrobial peptides have been purified from potato tubers. As an example, a 5 kDa pseudothion of S. tuberosum (Pth-St1) was found to be active against bacterial and fungal pathogens of potato such as Clavibacter michiganensis subspecies sepedonicus, Pseudomonas solanacearum, and Fusarium solani. Trypsin or insect α-amylase activities are not inhibited by Pth-St1 and it does not affect cell-free protein synthesis or β-glucuronidase activity with true thionins (Moreno et al., 1994). It was also found that Snakin-1 (stSN1) and Snakin-2 (stSN2) are active against the fungal pathogens, Clavibacter michiganensis subspecies sepedonicus and Botrytis cinerea at concentrations <10 μM (Kim et al., 2009). Patatin, a potato tuber storage protein, which was purified to homogeneity, has antioxidant and antiradical activity (Liu et al., 2003). Potamin-1 (PT-1), a stress-inducible trypsin inhibitor, was also present in potato tubers (Ledoigt et al., 2006). The potamin-1 (PT-1) trypsin-chymotrypsin protease inhibitor (5.6 kDa) was found to be thermostable without potential hemolytic activity but holds antimicrobial activity. It strongly inactivated the pathogenic microbial strains such as Candida albicans, Rhizoctonia solani, and Clavibacter michiganense sub sp. michiganinse (Kim et al., 2005).
Vicia faba L. (Field Bean)
A trypsin/chymotrypsin inhibitor with a molecular weight of 18 kDa was purified from seeds of Vicia faba L (Gupta et al., 2000). Fernandes and Banerji (1995) tested the ability of the FBPI, when administered by gavage, to subdue benzopyrene (BP)-induced neoplasia of the forestomach of mice. The mice that were treated with heat-inactivated FBPI showed similar tumor multiplicity to the BP-treated group, indicating that inhibitory capacity. These findings indicated the ability of FBPIs as effective chemo-protectors against gastric cancer in animals and, possibly, in humans as well. The purified FBPI has been found to be quite similar to the Soybean-derived BBI in its properties and anticarcinogenic potentials (Banerji and Fernandes, 1994; Fernandes and Banerji, 1995, 1996). Skin carcinogenesis can be effectively suppressed by topical treatment with a FBPI as reported by Fernandes and Banerji (1996) and plasmin inhibitory activity of FBPI can help to stop pulmonary metastasis of B16F10 melanoma cells systemically injected into BDF1 mice (Banerji et al., 1998a). Banerji et al. (1998b) investigated the ability of FBPI to stop ethylnittrosourea (ENU)-induced tumors of the nervous system of Sprague-Dawley rats. Murugesan et al. (2001) labeled the same FBPI with 99m to determine its capability to identify tumors in tumor-bearing rat models when a neural tumor incidence of 100% in the rats treated with heat-inactivated FBPI confirmed that the tumor suppressor activity of FBPI is related to its PI activity (Banerji et al., 1998b). This labeling was done with Sn2+ as a reducing agent and the yield was 95% which was stable for 2 h at ambient temperature. This study indicated that 99mTcFBPI has the exact prospective for imaging gliomas and probably other tumors as well. The study also indicated that it was possible to label FBPI with radionuclides such as 186Rh and 153Sm, which could also be used for the detection of tumors particularly of glial origin in patients.
Vigna unguiculata L. (Black-Eyed Pea)
The Black-eyed pea Trypsin/Chymotrypsin Inhibitor (BTCI) isolated and purified from Vigna unguiculata (Cowpea) seeds is a natural PPI, and it belongs to the BBI family with two different and independent reactive sites for trypsin (Lys26) and chymotrypsin (Phe53) (Souza et al., 2014). BTCI is a globular protein comprising 83 amino acid residues with seven disulfide bonds and molecular weight of 9.1 kDa (Barbosa et al., 2007).
The effects of the BTCI (Figure 4), on the MCF-7 breast cancer cells was reported by Joanitti et al. (2010). BTCI-induced apoptosis, cell death due to morphological alterations of the cell and lysosome membrane permeation demonstrated via cytostatic and cytotoxic findings. The anti-carcinogenic capabilities of BBI and newly identified BTCI established a promising tool for drug developments aimed to treat breast cancer. Recently, Souza et al. (2014) reported the effects of a BTCI on proteasome 20S in MCF-7 breast cancer cells and the catalytic activity of the purified 20S proteasome from horse erythrocytes, as well as the structural analysis of the BTCI-20S proteasome complex. Furthermore, Mehdad et al. (2016) stated that BTCI significantly decreased human breast adenocarcinoma cell viability by inhibiting the activity of proteasome 20S, with an associated cytostatic effect at the G2/M phase of the cell cycle and an increase in apoptosis.
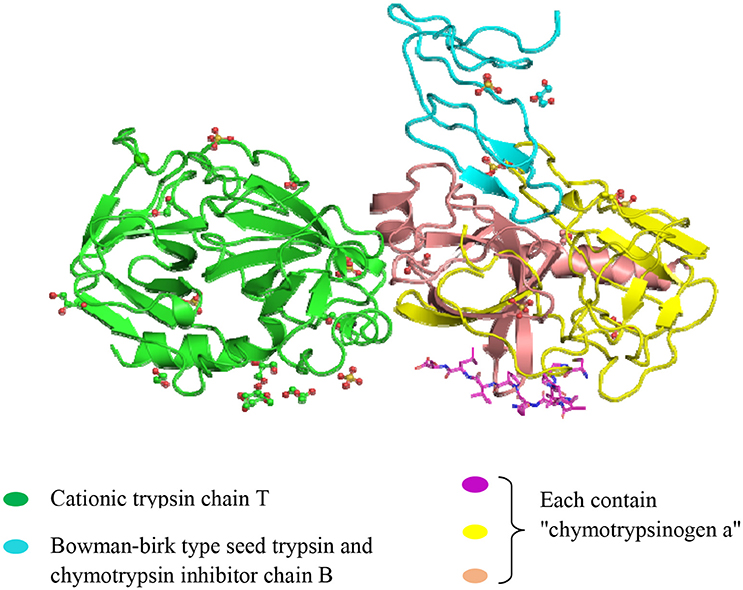
Figure 4. Crystal structure of the Bowman-Birk serine protease inhibitor BTCI in complex with a copy of cationic trypsin and three copies of chymotrypsinogen A. (http://www.ebi.ac.uk/pdbe-srv/view/entry/3ru4/summary.html).
Numerous protease inhibitors have been isolated and characterized from different plant species. PIs were isolated from Scopolia japonica cultured cells and these were found to have potential inhibitory activity against trypsin, chymotrypsin, kallikrein, plasmin, and pepsin but could not inhibit pain using synthetic or natural substrates (Sakato et al., 1975). Proteases like papain from Carica papaya L. has been used in different industrial processes including pharmaceutical process (Macalood et al., 2013). BBI like inhibitors were also isolated from sunflower (sunflower trypsin inhibitor-1; Luckett et al., 1999; Korsinczky et al., 2001; Marx et al., 2003; Lęgowska et al., 2010) and peanut (Arachis hypogaea L.; Tur Sinal et al., 1972; Norioka and Ikenaka, 1983; Suzuki et al., 1987). Protease inhibitors belonging to the Kunitz inhibitor family has been purified from pigeonpea (Cajanus cajan (L.)) PUSA 33 variety (Haq and Khan, 2003). A red gram protease inhibitor (RgPI) was extracted and purified from Cajanus cajan (Kollipara et al., 1994; Prasad et al., 2010b). BBI type inhibitors were purified and characterized from /in black gram (Vigna mungo (L.) Hepper) (Prasad et al., 2010a).
Mustard trypsin inhibitor was isolated and sequenced from the seeds of Sinapis alba L., members of the family Cruciferae. It was a serine protease inhibitor but did not show any structural similarity with other identified families of serine protease inhibitors from plants and it comprised more cysteine and glycine residues (Menegatti et al., 1985, 1992). In contrary, a similar type of PI was isolated and characterized from Brassica napus (rapeseed) (Ceciliani et al., 1994; Ascenzi et al., 1999). Inhibitors from Secale cereale L. and Ricinus communis L. also exhibited trypsin inhibitory activity (Odani et al., 1983). A few reports are available regarding the dual inhibitory activity (both serine proteases and α- amylase inhibitory activity) of some inhibitors from Zea mays L. (maize) (Filiz et al., 2014). Potato inhibitors II has been reported in flowers of Nicotiana alata Link & Otto (tobacco) (Atkinson et al., 1993). Serine protease inhibitors were reported in phloem sap of Cucurbita maxima (Kuroda et al., 2001), Arabidopsis and rice (Oryza sativa cv. Nipponbare; Fluhr et al., 2012) and pumpkin (Cucurbita maxima; Yoo et al., 2000). Squash Inhibitors have been reported in cucumber, zucchini (Wieczorek et al., 1985), watermelon (Citrullus vulgaris), spaghetti squash, red bryony (Bryonia dioica), figleaf gourd (Otlewski et al., 1987; Polanowski et al., 1987), Momordica repens (Joubert, 1984), wild cucumber (Cyclanthera pedata; Kuroda et al., 2001), wax gourd (Benincasa hispida (Thumb) cogn) (Atiwetin et al., 2006) and in several other plants of cucurbit family. The small size, structural rigidity and stability of the members of the squash inhibitor family serve as potential materials for studying serine protease-protein inhibitor interaction (Otlewski and Krowarsch, 1996). Cysteine protease inhibitors were reported in Rice (oryzacystatin) (Abe et al., 1987a,b), maize (Abe et al., 1992), apple fruit (Ryan et al., 1998), and several other monocotyledonous as well as dicotyledonous plants (Pernas et al., 1998; Sakuta et al., 2001). Aspartic protease inhibitor reported in Anchusa strigosa (Abuereish, 1998) and squash inhibit pepsin, which is a digestive aspartic protease.
Naturally-Occurring Plant Protease Inhibitors in Clinical Trials
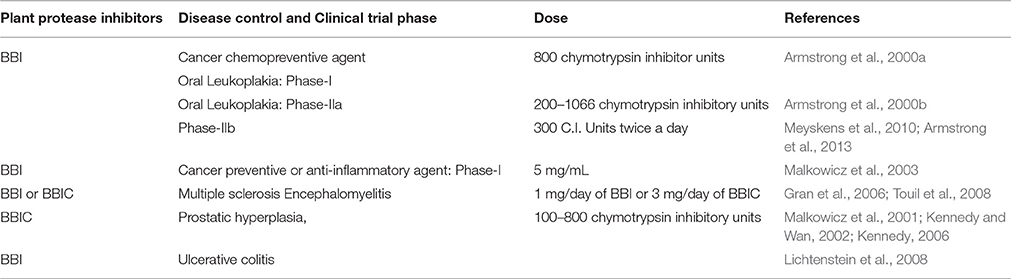
Many human clinical trials to assess the effect of BBIC have been finalized or are in progress (Armstrong et al., 2000b; Malkowicz et al., 2001; Lichtenstein et al., 2008). BBI clinical studies were carried out in patients (with the approval of Food and Drug Administration) with oral leukoplakia, prostrate cancer, gingivitis, ulcerative colitis, lung cancer, anti-inflammation (Figure 5), multiple sclerosis and encephalomyelitis using BBIC dose up to 1066 chymotrypsin inhibitory units (C.I.U) per day for single patient (Sugano, 2006; Table 2). During human trials, even though the BBI level could not be found in the blood after oral BBIC medicating, it could be detected in urine (Wan et al., 2000).
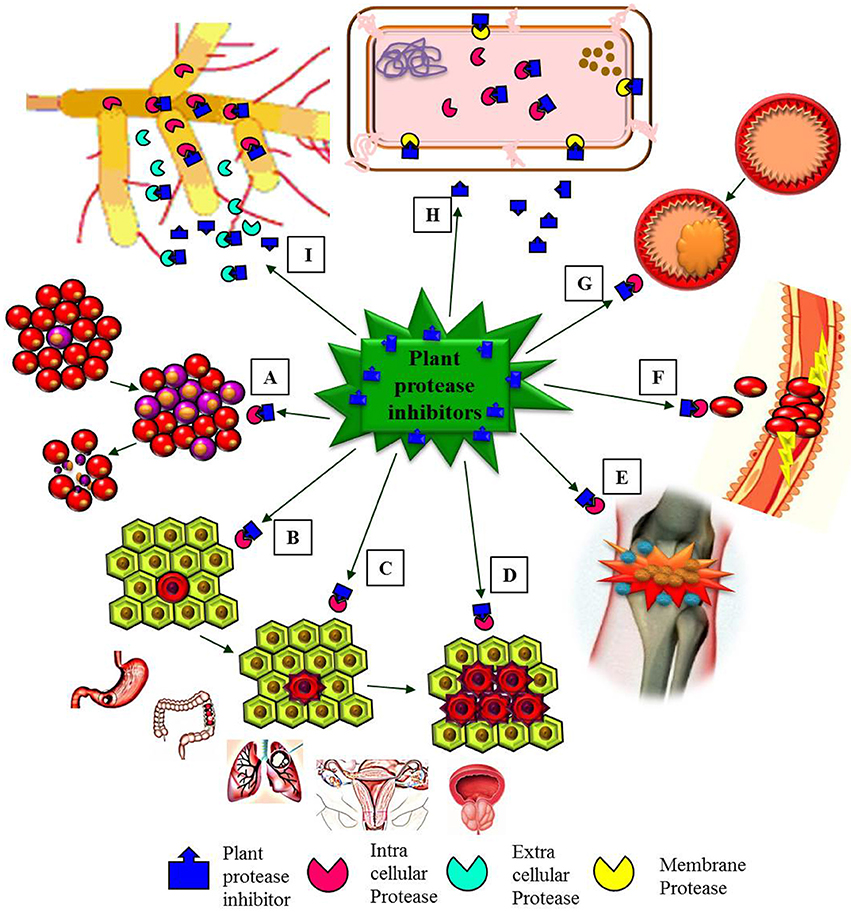
Figure 5. Schematic representation emphasizing major pharmacological effects of plant protease inhibitors (PPIs). (A) PPIs induces apoptosis of leukemia cells (healthy red blood cells and leukemia blood cells). (B–D) PPIs showed in vitro anti-carcinogenic effects in initiation (B), proliferation (C), and progression (D) stages of different types of cancers (gastric, colon, lung, ovarian, and prostate cancers). (E) PPIs as anti-inflammatory agents. (F) The role of PPIs as anti-coagulant. (G) PPIs active against cardiovascular diseases (plaque formation in a blood vessel). (H) Antibacterial activity of PPIs. (I). Antifungal activity of PPIs.
Until now, finished clinical trials did not show any toxicity or neutralizing antibodies against BBIC have been reported in patients. Armstrong et al. (2000a) reported the safety and nontoxic nature of BBI and BBIC in phase I trial of BBIC administered as an oral troche in patients with oral leukoplakia. The safety of BBIC has also been verified in other human clinical trials. The Phase-IIa trial of BBIC demonstrated simultaneous changes in neu protein levels and protease activity in patients treated with daily doses of 600 C.I.U of BBIC, administered as 300 C.I.U twice a day as 3 grams of BBIC (Armstrong et al., 2000b). This Phase-IIb clinical trial of BBIC did not show many significant differences between placebo and BBIC treatment. However, both BBIC treatment and placebo group showed statistically substantial decreases in total lesion area (Armstrong et al., 2013). Gran et al. (2006) found that BBIC administration to Lewis rats with experimental autoimmune encephalomyelitis (EAE) considerably suppressed the disease. Later, Touil et al. (2008) tested the purified BBI effects on clinical and histopathological responses of EAE in two models (relapsing/remitting EAE in SJL/J mice and chronic EAE in C57BL/6 mice). Treatment with BBI (1 mg/day of BBI or 3 mg/day of BBIC) in both EAE models significantly effected disease parameters such as onset, severity, weight loss, inflammation, and demyelination. Moreover, it significantly reduced the incidence of optic neuritis and prevented loss of retinal ganglion cells (Touil et al., 2008). Kennedy and Wan (2002) found that BBIC had a significant inhibitory effect on the growth and clonogenic survival of BRF-55T, 267B1/Ki-ras, LNCaP, and PC-3 cells. Dr. Kennedy's group and others carried out six human clinical trials for the following issues: cancer prevention on oral leukoplakia, treatment of benign prostatic hyperplasia, prostate cancer detection, and treatment, treatment of ulcerative colitis, gingivitis, or esophagitis (Malkowicz et al., 2001; Kennedy, 2006). A double-blind, randomized, phase I clinical trial was conducted in 19 males with Benign prostatic hyperplasia, which is an initial stage of prostate cancer and lower urinary tract symptoms (Malkowicz et al., 2001). This study demonstrated that BBIC treatment for 6 months reduced levels of prostate-specific antigen (PSA) which is a clinical marker for prostate cancer, and prostate volume in patients. Further clinical studies will be required to determine the potential of BBIC as prostate cancer chemopreventive agent. BBIC shows promise to become an effective nontoxic chemopreventive agent based on results of extensive preclinical studies, and Phase I and Phase IIa clinical trials. BBIC has dose-related clinical activity against oral leukoplakia and modulates levels of Neu (Neu immune histochemical staining intensity for lesions) and protease activity (Armstrong et al., 2003). Wan et al. (1999a) have reported a specific substrate hydrolysis methodology to measure the protease activity of human oral mucosal cells. Eventually authors have described the connection between neu oncogene expression and protease activity in patients that are registered for an oral cancer prevention trial using (BBIC) as the preventive agent. A completed clinical trial was performed to examine the safety and possible benefits of BBIC in patients with active ulcerative colitis (Lichtenstein et al., 2008). BBI demonstrated anti-inflammatory effects in patients with ulcerative colitis without toxicity. Investigation and identification of natural plant protease inhibitors and synthesis of peptidomimetic molecules have provided various beneficiary compounds that are successful in using among many human studies. Numerous plant protease inhibitors are undertaking an additional evaluation in human clinical trials.
Conclusion
New research strategies are now focusing on the understanding of protease-regulated cascades, along with a specific selection of targets and improved inhibitor specificity. Many protease inhibitors have been found in natural sources and also have been synthesized for clinical uses. Among all, soybean BBI represent the most expansively studied members of the Bowman-Birk family, but related BBI from other dicotyledonous legumes (including Cicer arietinum (chickpea), Phaseolus vulgaris (common bean), Lens culinaris (lentil), and Pisum sativum (pea)) and from monocotyledonous grasses (Poaceae) (including (Triticum aestivum) wheat, Oryza sativa (rice) and Hordeum vulgare (barley) species), has been well recognized and characterized. Many pharmaceutical companies are taking keen interests in several plant protease inhibitors, which are currently in human trials (Clemente et al., 2010; Clemente and Arques, 2014). The sequences and crystal structure of many plant protease inhibitors are now available. But, still only a few are used in medicine and are in clinical trials (Majumdar, 2013). There are several advantages of using plant protease inhibitors compared to synthetic ones. Plant protease inhibitors can also be supplied through diet (e.g., rice, potato, legumes, soybean, corn, cucurbits, cereals etc.) by adding some extra plant based food preparations which will have no side effects on human body. Extensive research has to be done to find out the possible candidates of protease inhibitors that have therapeutic importance from plants. With the growing population and diseases, there is a need to explore more plant protease inhibitors useful in the treatment and control of human diseases. This review illustrates the enormous potential of the protease inhibitors from plant species in medicine.
Author Contributions
ZC initiated the project. SS contributed to the figures. SS, ZC wrote the manuscript.
Funding
The authors thank the fund from Ministry of Education Singapore (RP 1/14 CZ).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abe, K., Emori, Y., Kondo, H., Suzuki, K., and Aria, S. (1987a). Molecular cloning of a cysteine proteinase inhibitor of rice (oryzacystatin)-Homology with animal cystatins and transient expression in the ripening process of rice seeds. J. Biol. Chem. 262, 16793–16797.
Abe, M., Abe, K., Kudora, M., and Arai, S. (1992). Corn Kernel cysteine proteinase inhibitor as novel cystatin superfamily member of plant origin. Eur. J. Biochem. 209, 933–937. doi: 10.1111/j.1432-1033.1992.tb17365.x
Abe, M., Kondo, H., and Arai, S. (1987b). Purification and characterization of a rice cysteine proteinase inhibitor. Agric. Biol. Chem. 51, 2763–2768.
Abuereish, G. M. (1998). Pepsin inhibitor from roots of Anchusa strigosa. Phytochemistry 48, 217–221. doi: 10.1016/S0031-9422(97)01131-X
Adlercreutz, C. H., Goldin, B. R., Gorbach, S. L., Höckerstedt, K. A., Watanabe, S. Hämäläinen, E. K., et al. (1995). Soybean phytoestrogen intake and cancer risk. J. Nutr. 125, 757S–770S.
Ammon, H. P. (2002). Boswellic acids (components of frankincense) as the active principle in treatment of chronic inflammatory disease. Wien. Med. Wochenschr. 152, 373–378. doi: 10.1046/j.1563-258X.2002.02056.x
Armstrong, W. B., Kennedy, A. R., Wan, X. S., Atiba, J., McLaren, C. E., and Meyskens, F. L. (2000a). Single-dose Administration of Bowman-Birk Inhibitor Concentrate in Patients with Oral Leukoplakia. Cancer. Epidemiol. Biomarkers. Prev. 9, 43–47.
Armstrong, W. B., Kennedy, A. R., Wan, X. S., Taylor, T. H., Nguyen, Q. A., Jensen, J., et al. (2000b). Clinical modulation of oral leukoplakia and protease activity by Bowman-Birk inhibitor concentrate in a phase IIa chemoprevention trial. Clin. Cancer. Res. 6, 4684–4691.
Armstrong, W. B., Taylor, T. H., Kennedy, A. R., Melrose, R. J., Messadi, D. V., et al. (2013). Bowman-birk inhibitor concentrate and Oral Leukoplakia: a randomized phase IIb trial. Cancer Prev. Res. (Phila.) 6, 410–418. doi: 10.1158/1940-6207.CAPR-13-0004
Armstrong, W. B., Wan, X. S., Kennedy, A. R., Taylor, T. H., and Meyskens, F. L. Jr. (2003). Development of the Bowman-Birk inhibitor for oral cancer chemoprevention and analysis of Neu immune histochemical staining intensity with Bowman-Birk inhibitor concentrate treatment. Laryngoscope 113, 1687–1702. doi: 10.1097/00005537-200310000-00007
Ascenzi, P., Ruoppolo, M., Amoresano, A., Pucci, P., Consonni, R., Zetta, L., et al. (1999). Characterization of low-molecular-mass trypsin isoinhibitors from oil-rape (Brassica napus var. oleifera) seed. Eur. J. Biochem. 261, 275–284. doi: 10.1046/j.1432-1327.1999.00275.x
Atiwetin, P., Harada, S., and Kamei, K. (2006). Serine protease inhibitor from Wax Gourd (Benincasa hispida [Thumb] seeds). Biosci. Biotech. Biochem. 70, 743–745. doi: 10.1271/bbb.70.743
Atkinson, A. H., Heath, R. L., Simpson, R. J., Clarke, A. E., and Anderson, M. A. (1993). Proteinase inhibitors in Nicotiana alata stigmas are derived from a precursor protein which is processed into five homologous inhibitors. Plant Cell 5, 203–213. doi: 10.1105/tpc.5.2.203
Banerjee, S., Li, Y., Wang, Z., and Sarkar, F. H. (2008). Multi-targeted therapy of cancer by genistein. Cancer. Lett. 269, 226–242. doi: 10.1016/j.canlet.2008.03.052
Banerji, A., Fernandes, A., and Bane, S. (1998b). Treatment with field bean protease inhibitor can effectively repress ethylnitrosourea (ENU)-induced neoplasms of the nervous system in Sprague-Dawley rats. Cancer. Lett. 130, 161–167. doi: 10.1016/S0304-3835(98)00135-9
Banerji, A., Fernandes, A., Bane, S., and Ahire, S. (1998a). The field bean protease inhibitor has the potential to suppress B16F10 melanoma cell lung metastasis in mice. Cancer. Lett. 129, 15–20. doi: 10.1016/S0304-3835(98)00090-1
Banerji, A. P., and Fernandes, A. O. (1994). Field bean protease inhibitor preparations, unlike methotrexate, can completely suppress Yoshida sarcoma tumor in rats. Cell. Biol. Int. 18, 1025–1034. doi: 10.1006/cbir.1994.1026
Barbosa, J. A. R. G., Silva, L. P., Teles, R. C. L., Esteves, G. F., Azevedo, R. B., et al. (2007). Crystal structure of the Bowman-Birk inhibitor from Vigna unguiculata Seeds in Complex with b-Trypsin at 1.55 a resolution and its structural properties in association with proteinases. Biophys. J. 92, 1638–1650. doi: 10.1529/biophysj.106.090555
Béliveau, R., and Gingras, D. (2007). Role of nutrition in preventing cancer. Can. Family. Phys. 53, 1905–1911.
Bijina, B., Chellappan, S., Krishna, J. G., Basheer, S. M., Elyas, K. K., Bahkali, A. H., et al. (2011). Protease inhibitor from Moringa oleifera with potential for use as therapeutic drug and as seafood preservative. Saudi. J. Bio. Sci. 18, 273–281. doi: 10.1016/j.sjbs.2011.04.002
Billings, P. C., Newberne, P. M., and Kennedy, A. R. (1990). Protease inhibitor suppression of colon and anal gland carcinogenesis induced by dimethylhydrazine. Carcinogenesis 11, 1083–1086. doi: 10.1093/carcin/11.7.1083
Birk, Y. (1961). Purification and some properties of a highly active inhibitor of trypsin and alpha-chymotrypsin from soybeans. Biochim. Biophys. Acta 54, 378–381. doi: 10.1016/0006-3002(61)90387-0
Block, E. (1992). The organosulfur chemistry of the genus Allium-Implications for the organic chemistry of sulfur. Angew. Chem. Int. Edn. Engl. 31, 1135–1178. doi: 10.1002/anie.199211351
Borodin, E. A., Pamirsky, I. E., Shtarberg, M. A., Dorovskikh, V. A., Korotkikh, A. V., Tarumizu, C., et al. (2013). “Effects of soybean trypsin inhibitor on hemostasis,” in Soybean - Bio-Active Compounds, ed H. A. El-Shemy (Rijeka: InTech).
Bowman, D. (1946). Differentiation of soybean antitryptic factors. Proc. Soc. Exp. Biol. Med. 63, 547–550. doi: 10.3181/00379727-63-15668
Caccialupi, P., Ceci, L. R., Siciliano, R. A., Pignone, D., Clemente, A., and Sonnante, G. (2010). Bowman-Birk inhibitors in lentil: Heterologous expression, functional characterisation and anti-proliferative properties in human colon cancer cells. Food. Chem. 120, 1058–1066. doi: 10.1016/j.foodchem.2009.11.051
Caceres, A., Cabrera, O., Morales, O., Mollinedo, P., and Mendia, P. (1991). Pharmacological properties of Moringa oleifera: preliminary screening for antimicrobial activity. J. Ethnol. Pharmacol. 33, 213–216. doi: 10.1016/0378-8741(91)90078-R
Campos, F. A. P., and Richardson, M. (1983). The complete amino acid sequence of the bifunctional-amylase/trypsin inhibitor from seeds of ragi (Indian finger millet, Eleusine coracana Gaertn.). FEBS Letters 152, 300–304. doi: 10.1016/0014-5793(83)80400-1
Campos, J., Mart'ınez-Gallardo, N., Mendiola-Olaya, E., and Blanco-Labra, A. (1997). Purification and partial characterization of a proteinase inhibitor from Tepary bean (Phaseolus acutifolius) seeds. J. Food. Biochem. 21, 203–218. doi: 10.1111/j.1745-4514.1997.tb00215.x
Capaldi, S., Perduca, M., and Faggion, B. (2007). Crystal structure of the anticarcinogenic Bowman-Birk inhibitor from snail medic (Medicago scutellata) seeds complexed with bovine trypsin. J. Struct. Biol. 158, 71–79. doi: 10.1016/j.jsb.2006.10.017
Catalano, C. M., Czymmek, K. J., Gann, J. G., and Sherrier, D. J. (2007). Medicago truncatula syntaxin SYP132 defines the symbiosome membrane and infection droplet membrane in root nodules. Planta 225, 541–550. doi: 10.1007/s00425-006-0369-y
Catalano, M., Ragona, L., Molinari, H., Tava, A., and Zetta, L. (2003). Anticarcinogenic Bowman Birk inhibitor isolated from snail medic seeds (Medicago scutellata): solution structure and analysis of self-association behaviour. Biochemistry 42, 2836–2846. doi: 10.1021/bi020576w
Ceciliani, F., Bortolotti, F., Menegatti, E., Ronchi, S., Ascenzi, P., and Palmieri, S. (1994). Purification, inhibitory properties, amino acid sequence and identification of the reactive site of a new serine proteinase inhibitor fromoil-rape (it Brassica napus) seed. FEBS. Lett. 342, 221–224. doi: 10.1016/0014-5793(94)80505-9
Chan, A. T., Baba, Y., Sima, K., Nosho, K., Chung, D. C., Hung, K. E., et al. (2010). Cathepsin B expression and survival in colon cancer: implications for molecular detection of Neoplasia. Cancer. Epidemiol. Biomarkers. Prev. 19, 2777–2785. doi: 10.1158/1055-9965.EPI-10-0529
Cheenpracha, S., Park, E. J., Yoshida, W. Y., Barit, C., Wall, M., Pezzuto, J. M., et al. (2010). Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleiferafruits. Bio. Organ. Med. Chem. 18, 6598–6602. doi: 10.1016/j.bmc.2010.03.057
Chen, Y. W., Huang, S. C., Lin-Shiau, S. Y., and Lin, J. K. (2005). Bowman-Birk inhibitor abates proteasome function and suppresses the proliferation of MCF7 breast cancer cells through accumulation of MAP kinase phosphatase-1. Carcinogenesis 26, 1296–1306. doi: 10.1093/carcin/bgi062
Clemente, A., and Arques, M. C. (2014). Bowman-Birk inhibitors from legumes as colorectal chemopreventive agents. World. J. Gastrol. Enterol. 20, 10305–10315. doi: 10.3748/wjg.v20.i30.10305
Clemente, A., Carmen Marín-Manzano, M., Jiménez, E., Carmen Arques, M., and Domoney, C. (2012). The anti-proliferative effect of TI1B, a major Bowman-Birk isoinhibitor from pea (Pisumsativum L.), on HT29 colon cancer cells is mediated through protease inhibition. Br. J. Nutr. 1, S135–S144. doi: 10.1017/S000711451200075X
Clemente, A., Gee, J. M., Johnson, I. T., Mackenzie, D. A., and Domoney, C. (2005). Pea (Pisum sativum L.) protease inhibitors from the Bowman-Birk class influence the growth of human colorectal adenocarcinoma HT29 cells in vitro. J. Agric. Food. Chem. 53, 8979–8986. doi: 10.1021/jf051528w
Clemente, A., Moreno, F. J., Marín-Manzano, M. C., Jiménez, E., and Domoney, C. (2010). The cytotoxic effect of Bowman-Birk isoinhibitors, IBB1 and IBBD2, from soybean (Glycine max) on HT29human colorectal cancer cells is related to their intrinsic ability to inhibit serine proteases. Mol. Nutr. Food. Res. 54, 396–405. doi: 10.1002/mnfr.200900122
Clemente, A., Sonnante, G., and Domoney, C. (2011). Bowman-Birk inhibitors from legumes and human gastro intestinal health: current status and perspectives. Curr. Protein. Pept. Sci. 12, 358–373. doi: 10.2174/138920311796391133
Constabel, F. (1990). Medicinal plant biotechnology. Planta Med. 56, 421–425. doi: 10.1055/s-2006-961002
Cooreman, W. M., Scharpe, S., Demeester, J., and Lauwers, A. (1976). Bromelain, biochemical and pharmacological properties. Pharm. Acta. Helv. 4, 73–79.
Correa, P. (1981). Epidemiologic correlations between diet and cancer frequency. Cancer. Res. 41, 3685–3690.
Dahl, S. W., Rasmussen, S. K., and Hejgaard, J. (1996a). Heterologous expression of three plant serpins with distinct inhibitory specificities. J. Biol. Chem. 271, 25083–25088. doi: 10.1074/jbc.271.41.25083
Dahl, S. W., Rasmussen, S. K., Petersen, L. C., and Hejgaard, J. (1996b). Inhibition of coagulation factors by recombinant barley serpin BSZX. FEBS. Letter. 394, 165–168. doi: 10.1016/0014-5793(96)00940-4
Darshan, S., and Doreswamy, R. (2004). Patented anti- inflammatory plant drug development from traditional medicine. Phyto. Ther. Res. 18, 343–357. doi: 10.1002/ptr.1475
De Feo, V. (1992). Medicinal and magical plants in the northern Peruvian Andes. Fito. Terapia. 53, 417–440.
de Kok, T. M., van Breda, S. G., and Manson, M. M. (2008). Mechanisms of combined action of different chemopreventive dietary compounds: a review. Eur. J. Nutr. 47, 51–59. doi: 10.1007/s00394-008-2006-y
De Leo, F., Volpicella, M., Licciulli, F., Liuni, S., Gallerani, R., and CeciL, R. (2002). PLANT-PIs: a database for plant protease inhibitors and their genes. Nucleic. Acids Res. 30, 347–348. doi: 10.1093/nar/30.1.347
de Paula, C. A., de Abreu Vieira, P. M., Silva, K. T., de Sá Cota, R. G., Carneiro, C. M., Castro-Borges, M., et al. (2012b). Bowman-Birk inhibitors, proteasome peptidase activities and colorectalpre neoplasias induced by 1,2-dimethylhydrazine in Swiss mice. Food. Chem. Toxicol. 50, 1405–1412. doi: 10.1016/j.fct.2012.01.036
de Paula, C. A., Coulson-Thomas, V. J., Ferreira, J. G., Maza, P. K., Suzuki, E., Nakahata, A. M., et al. (2012a). Enterolobium contortisiliquum trypsin inhibitor (EcTI), a plant proteinase inhibitor, decreases in vitro cell adhesion and invasion by inhibition of Src protein-focal adhesion kinase (FAK) signalling pathways. J. Biol. Chem. 287, 170–182. doi: 10.1074/jbc.M111.263996
Dirsch, V. M., and Vollmar, A. M. (2001). Ajoene, a non-steroidal anti-inflammatory drug (NSAID) – like properties? Biochem. Pharmacol. 61, 587–593. doi: 10.1016/S0006-2952(00)00580-3
Dunaevsky, Y. E., Gladysheva, I. P., Pavlukova, E. B., Beliakova, G. B., Gladyshev, D. P., Papisova, A. I., et al. (1997). The anionic protease BWI-1 from buckwheat seeds. Kinetic properties and possible roles. Physiol. Plant. 101, 483–488. doi: 10.1111/j.1399-3054.1997.tb01027.x
Fernanda Troncoso, M., Cerdá Zolezzi, P., Hellman, U., and Wolfenstein-Todel, C. (2003). A novel trypsin inhibitor from Peltophorum dubium seeds, with lectin-like properties, triggers rat lymphoma cell apoptosis. Arch. Biochem. Biophys. 411, 93–104. doi: 10.1016/S0003-9861(02)00726-9
Fernandes, A. O., and Banerji, A. P. (1995). Inhibition of benzopyrene-induced forestomach tumors by field bean protease inhibitor (s). Carcinogenesis 16, 1843–1846. doi: 10.1093/carcin/16.8.1843
Fernandes, A. O., and Banerji, A. P. (1996). The field bean protease inhibitor can effectively suppress 7,12-dimethylbenz[a]anthracene-induced skin tumorigenesis in mice. Erratum in: Cancer. Lett. 106, 145.
Ferreira, J. G., Diniz, P. M., de Paula, C. A., Lobo, Y. A., Paredes-Gamero, E. J., Paschoalin, T., et al. (2013). The impaired viability of prostate cancer cell lines by the recombinant plant kallikrein inhibitor. J. Biol. Chem. 288, 13641–13654. doi: 10.1074/jbc.M112.404053
Fields, C., Mallee, P., Muzard, J., and Lee, G. U. (2012). Isolation of Bowman-Birk-Inhibitor from soybean extracts using novel peptide probes and high gradient magnetic separation. Food. Chem. 134, 1831–1838. doi: 10.1016/j.foodchem.2012.03.085
Filiz, E., Tombuloglu, H., Koc, I., and Osma, E. (2014). Characterization of Wound_Induced Serine Protease Inhibitor (wip1) Genes and Proteins in Turkish Maize Varieties. Biochem. (Mos.) 79, 836–844. doi: 10.1134/S0006297914080124
Fluhr, R., Lampl, N., and Roberts, T. H. (2012). Serpin protease inhibitors in plant biology. Physiol. Plant. 145, 95–102. doi: 10.1111/j.1399-3054.2011.01540.x
García-Gasca, T., García-Cruz, M., Hernandez-Rivera, E., López-Matínez, J., Casta-eda-Cuevas, L. A., Yllescas-Gasca, L., et al. (2012). Effects of Tepary Bean (Phaseolus acutifolius) Protease Inhibitor and Semipure Lectin Fractions on Cancer Cells. Nutr. Cancer. 64, 1269–1278. doi: 10.1080/01635581.2012.722246
Gautam, S. S., Mishra, S. K., Dash, V., Goyal, A. K., and Rath, G. (2010). Comparative study of extraction, purification and estimation of bromelain from stem and fruit of pineapple plant. Thai. J. Pharm. 34, 67–76.
Gran, B., Tabibzadeh, N., Martin, A., Ventura, E. S., Ware, J. H., Zhang, G. X., et al. (2006). The protease inhibitor, Bowman-Birk Inhibitor, suppresses experimental autoimmune encephalomyelitis: a potential oral therapy for multiple sclerosis. Mult. Scler. 12, 688–697. doi: 10.1177/1352458506070769
Grzonka, Z., Kasprzykowski, F., and Wiczk, W. (2007). “Cysteine proteases,” in Industrial Enzymes, eds J. Polaina and P. MacCabe (New York, NY: Springer), 181–195.
Gupta, P., Dhawan, K., Malhotra, S. P., and Singh, R. (2000). Purification and characterization of trypsin inhibitor from seeds of faba bean (Vicia faba L.). Acta Physiol. Plant. 22, 433–438. doi: 10.1007/s11738-000-0085-3
Hale, L. P., Greer, P. K., Trinh, C. T., and James, C. L. (2005). Proteinase activity and stability of natural bromelain preparations. Int. Immunol. Pharmacol. 5, 783–793. doi: 10.1016/j.intimp.2004.12.007
Haq, S. K., and Khan, R. H. (2003). Characterization of a proteinase inhibitor from Cajanus cajan (L.). J. Protein. Chem. 22, 543–554. doi: 10.1023/B:JOPC.0000005504.57372.5b
Hasler, C. M. (1998). Functional foods: their role in disease prevention and health promotion. Food. Technol. 52, 63–70.
Hatano, K., Kojimam, M., Tanokuram, M., and Takahashi, K. (1996). Solution structure of bromelain inhibitor IV from pineapple stem: structural similarity with Bowman-Birk trypsin/chymotrypsin inhibitor from soybean. Biochemistry 35, 5379–5384. doi: 10.1021/bi952754+
Hejgaard, J., Rasmussen, S. K., Brandt, A., and Svendsen, I. (1985). Sequence homology between barley endosperm protein Z and protease inhibitors of the α1-antitrypsin family. FEBS Lett. 180, 89–94. doi: 10.1016/0014-5793(85)80238-6
Hernández-Ledesma, B., Hsieh, C. C., and De Lumen, B. O. (2009). Lunasin, A novel seed peptide for cancer prevention. Peptides 30, 426–430. doi: 10.1016/j.peptides.2008.11.002
Hill, A. J., Peikin, S. R., Ryan, C. A., and Blundell, J. E. (1990). Oral administration of proteinase inhibitor II from potatoes reduces energy intake in man. Physiol. Behav. 48, 241–246. doi: 10.1016/0031-9384(90)90307-P
Hsieh, C. C., Hernández-Ledesma, B., Jeong, H. J., Park, J. H., and de Lumen, B. O. (2010). Complementary roles in cancer prevention: protease inhibitor makes the cancer preventive peptide lunasin bioavailable. PLoS ONE 5:e8890. doi: 10.1371/journal.pone.0008890
Huang, G. J., Sheu, M. J., Chen, H. J., Chang, Y. S., and Lin, Y. H. (2007). Growth inhibition and induction of apoptosis in NB4 promyelocytic leukemia cells by trypsin inhibitor from sweet potato storage roots. J. Agric. Food. Chem. 55, 2548–2553. doi: 10.1021/jf063008m
Izaka, K. I. M., Yamada, M., Kawano, T., and Suyama, T. (1972). Gastrointestinal absorption and anti-inflammatory effect of bromelain. Jpn. J. Pharmecol. 4, 519–534. doi: 10.1254/jjp.22.519
Jaber, R. (2002). Respiratory and allergic diseases: from upper respiratory tract infections to asthma. Prim. Care. 2, 231–261. doi: 10.1016/S0095-4543(01)00008-2
Joanitti, G. A., Azevedo, R. B., and Freitas, S. M. (2010). Apoptosis and lysosome membrane permeabilization induction on breast cancer cells by an anticarcinogenic Bowman–Birk protease inhibitor from Vigna unguiculata seeds. Cancer. Lett. 293, 73–81. doi: 10.1016/j.canlet.2009.12.017
Joubert, F. J. (1984). Trypsin Inhibitors from Momordica Repens Seeds. Phyto. Chem. 23, 1401–1406. doi: 10.1016/S0031-9422(00)80474-4
Joubert, F. J., Kruger, H., Townshend, G. S., and Botes, D. P. (1979). Purification, some properties and the complete primary structures of two protease inhibitors (DE-3 and DE-4) from Macrotyloma axillare seed. Eur. J. Biochem. 97, 85–91. doi: 10.1111/j.1432-1033.1979.tb13088.x
Kelly, S. (1996). Bromelain: a literature review and discussion of its therapeutic applications. Altern. Med. Rev. 1, 243–257.
Kennedy, A. R. (2006). “The status of human trials utilizing Bowman-Birk Inhibitor Concentrate from soybeans,” in Soy in Health and Disease Prevention, ed M. Sugano (Boca Raton, FL: CRC Press; Taylor & Francis Group LLC).
Kennedy, A. R., Billings, P. C., Wan, X. S., and Newberne, P. M. (2002). Effects of Bowman-Birk Inhibitor on Rat Colon Carcinogenesis. Nutr. Cancer 43, 174–186. doi: 10.1207/S15327914NC432_8
Kennedy, A. R., and Wan, X. S. (2002). Effects of the Bowman-Birk inhibitor on growth, invasion, and clonogenic survival of human prostate epithelial cells and prostate cancer cells. Prostate 50, 125–133. doi: 10.1002/pros.10041
Kim, J. Y., Park, S. C., Hwang, I., Cheong, H., Nah, J. W., Hahm, K. S., et al. (2009). Protease inhibitors from plants with antimicrobial activity. Int. J. Mol. Sci. 10, 2860–2872. doi: 10.3390/ijms10062860
Kim, J. Y., Park, S. C., Kim, M. H., Lim, H. T., Park, Y., and Hahm, K. S. (2005). Antimicrobial activity studies on a trypsin-chymotrypsin protease inhibitor obtained from potato. Biochem. Biophys. Res Commun. 330, 921–927. doi: 10.1016/j.bbrc.2005.03.057
Kobayashi, H., Suzuki, M., Kanayama, N., and Terao, T. (2004). A soybean Kunitz trypsin inhibitor suppresses ovarian cancer cell invasion by blocking urokinase up regulation. Clin. Exp. Metastas. 21, 159–166. doi: 10.1023/B:CLIN.0000024751.73174.c2
Koepke, J., Ermler, U., Warkentin, E., Wenzl, G., and Flecker, P. J. (2000). Crystal structure of cancer chemopreventive Bowman-Birk inhibitor in ternary complex with bovine trypsin at 2.3 A resolution. Structural basis of Janus-faced serine protease inhibitor specificity. J. Mol. Biol. 298, 477–491. doi: 10.1006/jmbi.2000.3677
Kollipara, K. P., Singh, L., and Hymowitz, T. (1994). Genetic variation of trypsin and chymotrypsin inhibitors in pigeonpea [Cajanus cajan (L.) Millsp.] and its wild relatives. Theor. Appl. Genet. 88, 986–993. doi: 10.1007/BF00220806
Korsinczky, M. L., Schirra, H. J., Rosengren, K. J., West, J., Condie, B. A., Otvos, L., et al. (2001). Solution structures by 1H NMR of the novel cyclic trypsin inhibitor SFTI-1 from sunflower seeds and an acyclic permutant. J. Mol. Biol. 311, 579–591. doi: 10.1006/jmbi.2001.4887
Kumar, S., Hemavathi, A. B., and Hebbar, H. U. (2011). Affinity based reverse micellar extraction and purification of bromelain from pineapple (Ananascomosus L. Merryl) waste. Process. Biochem. 46, 1216–1220. doi: 10.1016/j.procbio.2011.02.008
Kuroda, M., Kiyosaki, T., Matsumoto, I., Misaka, T., Arai, S., and Abe, K. (2001). Molecular cloning, characterization and expression of wheat cystatins. Biosci. Biotechnol. Biochem. 65, 22–28. doi: 10.1271/bbb.65.22
Lanza, A., Tava, A., Catalano, M., Ragona, L., Singuaroli, I., Robustelli della Cuna, F. S., et al. (2004). Effects of the Medicago scutellata Trypsin Inhibitor (MsTI) on Cisplatin-induced Cytotoxicity in Human Breast and Cervical Cancer Cells. Anticancer. Res. 24, 227–234.
Laskowski, M. Jr., and Qasim, M. A. (2000). What can the structures of enzyme-inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochim. Biophysic. 1477, 324–337. doi: 10.1016/S0167-4838(99)00284-8
Ledoigt, G., Griffaut, B., Debiton, E., Vian, C., Mustel, A., Evray, G., et al. (2006). Analysis of secreted protease inhibitors after water stress in potato tubers. Int. J. Biol. Macromols. 38, 268–271. doi: 10.1016/j.ijbiomac.2006.03.016
Lęgowska, A., Lesner, A., Bulak, E., Jaśkiewicz, A., Sieradzan, A., Cydzik, M., et al. (2010). Inhibitory activity of double-sequence analogues of trypsin inhibitor SFTI-1 from sunflower seeds: an example of peptide splicing. FEBS. J. 277, 2351–2359. doi: 10.1111/j.1742-4658.2010.07650.x
Lemay, M., Murray, M. A., Davies, A., Roh-Schmidt, H., and Randolph, R. K. (2004). In vitro and ex vivo cyclooxygenase inhibition by a hops extract. Asian. Pac. J. Clin. Nutr. 13, S110.
Lichtenstein, G. R., Deren, J., Katz, S., Lewis, J. D., Kennedy, A. R., and Ware, J. H. (2008). Bowman-birk inhibitor concentrate: a novel therapeutic agent for patients with active ulcerative colitis. Dig. Dis. Sci. 53, 175–180. doi: 10.1007/s10620-007-9840-2
Li de la Sierra, I., Quillien, L., Flecker, P., Gueguen, J., and Brunie, S. (1999). Dimeric crystal structure of a Bowman-Birk protease inhibitor from pea seeds. J. Mol. Biol. 285, 1195–1207. doi: 10.1006/jmbi.1998.2351
Lim, T. K. (2013). Edible Medicinal and Non-medicinal Plants, Vol. 5, Fruits. Springer; Fagopyrum; Esculentum.
Lingaraju, M. H., and Gowda, L. R. (2008). A Kunitz trypsin inhibitor of Entada scandens seeds: another member with single disulfide bridge. Biochim. Biophys. Acta 1784, 850–855. doi: 10.1016/j.bbapap.2008.02.013
Liu, Y. W., Han, C. H., Lee, M. H., Hsu, F. L., and Hou, W. C. (2003). Patatin, the tuber storage protein of potato (Solanum tuberosum L.) exhibits antioxidant activity in vitro. J. Agric. Food. Chem. 51, 4389–4393. doi: 10.1021/jf030016j
Lizcano, L. J., Bakkali, F., Ruiz-Larrea, M. B., and Ruiz-Sanz, J. I. (2010). Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use. Food. Chem. 119, 1566–1570. doi: 10.1016/j.foodchem.2009.09.043
Losso, J. N. (2008). The biochemical and functional food properties of the Bowman-Birk Inhibitor. Critic. Rev. Food. Sci. Nutri. 48, 94–118. doi: 10.1080/10408390601177589
Luckett, S., Garcia, R. S., Barker, J. J., Konarev, A. V., Shewry, P. R., Clarke, A. R., et al. (1999). High-resolution structure of a potent, cyclic proteinase inhibitor from sunflower seeds. J. Mol. Biol. 290, 525–533. doi: 10.1006/jmbi.1999.2891
Macalood, S. J., Helen, J., Vicente, H. J., Renato, D., Boniao, R. D., Gorospe, J. G., et al. (2013). Chemical analysis of Carica papaya L. Crude Latex. Am. J. Plant. Sci. 4, 1941–1948. doi: 10.4236/ajps.2013.410240
Magee, P. J., Owusu-Apenten, R., McCann, M. J., Gill, C. I., and Rowland, I. R. (2012). Chickpea (Cicer arietinum) and other plant-derived protease inhibitor concentrates inhibit breast and prostate cancer cell proliferation in vitro. Nutr. Cancer 64, 741–748. doi: 10.1080/01635581.2012.688914
Mahajan, S. G., and Mehta, A. A. (2010). Immunosuppressive activity of ethanolic extract of seeds of Moringa oleifera Lam. in experimental immune inflammation. J. Ethnopharmacol. 130, 183–186. doi: 10.1016/j.jep.2010.04.024
Majumdar, D. D. (2013). Recent updates on pharmaceutical potential of plant protease inhibitors. Int. J. Med. Pharm. Sci. 3, 101–120.
Malkowicz, S. B., Liu, S.-P., Broderick, G, A., Wein, A. J., Kennedy, A. R., and Levin, R. M. (2003). Effect of the Bowman-Birk inhibitor (a soy protein) on in vitro bladder neck/urethral and penile corporal smooth muscle activity. Neurourol. Urodyn. 22, 54–57. doi: 10.1002/nau.10071
Malkowicz, S. B., McKenna, W. G., Vaughn, D. J., Wan, X. S., Propert, K. J., Rockwell, K., et al. (2001). Effects of Bowman-Birk Inhibitor Concentrate (BBIC) in Patients with Benign Prostatic Hyperplasia. Prostate 48, 16–28. doi: 10.1002/pros.1077
Mark, M., and Barness, S. (1991). The role of soy products in reducing risk of cancer. J. Natl. Cancer. Inst. 83, 8.
Marx, U. C., Korsinczky, M. L., Schirra, H. J., Jones, A., Condie, B., Otvos, L. Jr., et al. (2003). Enzymatic cyclization of a potent bowman-birk protease inhibitor, sunflower trypsin inhibitor-1, and solution structure of an acyclic precursor peptide. J. Biol. Chem. 278, 21782–21789. doi: 10.1074/jbc.M212996200
Maskos, K., Huber-Wunderlich, M., and Glockshuber, R. (1996). RBI, a one-domain alpha-amylase/trypsin inhibitor with completely independent binding sites. Federat. Eur. Biochem. Soc. Lett. 397, 11–16. doi: 10.1016/S0014-5793(96)01131-3
Maurer, H. R. (2001). Bromelain: biochemistry, pharmacology and medical use. Cell. Mol. Life. Sci. 58, 1231–1245. doi: 10.1007/PL00000936
McCormick, D. L., Johnson, W. D., Bosland, M. C., Lubet, R. A., and Steele, V. E. (2007). Chemoprevention of Rat Prostate Carcinogenesis by Soy Isoflavones and Bowman-Birk Inhibitor. Nutr. Cancer 57, 184–193. doi: 10.1080/01635580701277478
Mehdad, A., Brumana, G., Souza, A. A., Barbosa, J. A. R. G., Ventura, M. M., and de Freitas, S. M. (2016). A Bowman–Birk inhibitor induces apoptosis in human breast adenocarcinoma through mitochondrial impairment and oxidative damage following proteasome 20S inhibition. Cell Death Discovery. 2, 15067. doi: 10.1038/cddiscovery.2015.67
Menegatti, E., Palmieri, S., Walde, P., and Luisi, P. L. (1985). Isolation and characterization of a trypsin inhibitorfrom white mustard (Sinapis alba L.). J. Agric. Food. Chem. 33, 784–789. doi: 10.1021/jf00065a003
Menegatti, E., Tedeschi, G., Ronchi, S., Bortolotti, F., Ascenzi, P., Thomas, R. M., et al. (1992). Purification, inhibitory properties and amino acid sequence of a new serine proteinase inhibitor from white mustard (Sinapis alba L.) seed. FEBS. Lett. 301, 10–14. doi: 10.1016/0014-5793(92)80199-Q
Meyskens, F. L., Taylor, T., Armstrong, W., Kong, L., Gu, M., Gonzalez, R., et al. (2010). Phase IIb randomized clinical chemoprevention trial of a Soybean-derived Compound (Bowman-Birk Inhibitor Concentrate) for Oral Leukoplakia. Cancer. Prev. Res. 3, CN02–05. doi: 10.1158/1940-6207.prev-10-cn02-05
Moreno, M., Segura, A., and Garcia-Olmedo, F. (1994). Pseudothionin-Sth1, a potato peptide active against potato pathogens. Eur. J. Biochem. 223, 135–139. doi: 10.1111/j.1432-1033.1994.tb18974.x
Murugesan, S., Banerji, A. P., Noronha, O. P., Samuel, A. M., and Fernandes, A. O. (2001). 99mTc-labeled field bean protease inhibitor can function as an efficient tumor detecting agent. Indian. J. Exp. Biol. 39, 742–747.
Mutlu, A., and Gal, S. (1999). Plant aspartic proteinases: enzymes on the way to a function. Physiol. Plant. 105, 569–576. doi: 10.1034/j.1399-3054.1999.105324.x
Nakahata, A. M., Bueno, N. R., Rocha, H. A., Franco, C. R., Chammas, R., Nakaie, C. R., et al. (2006). Structural and inhibitory properties of a plant proteinase inhibitor containing the RGD motif. Int. J. Biol. Macromol. 40, 22–29. doi: 10.1016/j.ijbiomac.2006.05.008
Nakahata, A. M., Mayer, B., Rie, C., de Paula, C. A., Karow, M., Neth, P., et al. (2011). The effects of a plant proteinase inhibitor from Enterolobium contortisiliquum on human tumor cell lines. Biol. Chem. 392, 327–336. doi: 10.1515/bc.2011.031
Neuhof, C., Oliva, M. L., Maybauer, D., Maybauer, M., Oliveira, C. D., Sampaio, M. U., et al. (2003). Effect of plant kunitz inhibitors from Bauhinia bauhinioides and Bauhinia rufa on pulmonary edema caused by activated neutrophils. Biol. Chem. 384, 939–944. doi: 10.1515/BC.2003.105
Norioka, S., and Ikenaka, T. (1983). Amino acid sequence of trypsin chymotrypsin inhibitors (A-I, A-II, B-I and B-II) from peanut (Arachishypogaea). A discussion on the molecular evolution of legume Bowman-Birk type inhibitor. J. Biochem. 94, 589–599.
Odani, S., and Ikenaka, T. (1973). Studies on soybean trypsin inhibitors. 8. Disulfide bridges in soybean Bowman-Birk proteinase inhibitor. J. Biochem. 74, 697–715.
Odani, S., Koide, T., Ono, T., and Ohnishi, K. (1983). Structural relationship between barley (Hordeum vulgare) trypsin inhibitor and castor-bean (Ricinus communis) storage protein. J. Biochem. 213, 543–545. doi: 10.1042/bj2130543
Oliva, M. L., Andrade, S. A., Batista, I. F., Sampaio, M. U., Juliano, M., Fritz, H., et al. (1999). Human plasma kallikrein and tissue kallikrein binding to a substrate based on the reactive site of a factor Xa inhibitor isolated from Bauhiniaungulata seeds. Immunopharmacology 45, 145–149. doi: 10.1016/S0162-3109(99)00146-0
Oliva, M. L. V., and Sampaio, M. U. (2008). Bauhinia Kunitz-type proteinase inhibitors: structural characteristics and biological properties. Biol. Chem. 389, 1007–1013. doi: 10.1515/BC.2008.119
Oliva, M. L., Silva, M. C., Sallai, R. C., Brito, M. V., and Sampaio, M. U. (2010). A novel subclassification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie 92, 1667–1673. doi: 10.1016/j.biochi.2010.03.021
Oliva, M. L., Ferreira, Rda. S., de Paula, C. A., Salas, C. E., and Sampaio, M. U. (2011). Structural and functional properties of kunitz proteinase inhibitors from leguminosae. Aminirev. Curr. Protein Pept. Sci. 12, 348–357. doi: 10.2174/138920311796391061
Otlewski, J., and Krowarsch, D. (1996). Squash inhibitor family of serine proteases. Acta Biochim. Polonica. 43, 431–444.
Otlewski, J., Whatley, H., Polanowski, A., and Wilusz, T. (1987). Amino acid sequences of trypsin inhibitors from watermelon (Citrullus vulgaris) and red bryony (Bryonia dioica). Biol. Chem. Hoppe-Seyler. 368, 1505–1507. doi: 10.1515/bchm3.1987.368.2.1505
Park, S. S., and Obha, H. (2004). Suppressive activity of protease inhibitors from buckwheat seeds against human T-Acute lymphoblastic leukemia cell lines. Appl. Biochem. Biotechnol. 117, 65–74. doi: 10.1385/ABAB:117:2:065
Pernas, M., Sanchez, M. R., Gomez, L., and Salcedo, G. (1998). A chestnut seed cystatin differentially effective against cysteine proteinases from closely related pests. Plant. Mol. Biol. 38, 1235–1242. doi: 10.1023/A:1006154829118
Pietta, P. G. (2000). Flavonoids as antioxidants. J. Nat. Prod. 63, 1035–1042. doi: 10.1021/np9904509
Plate, N. A., Valuev, L. I., Valueva, T. A., and Chupov, V. V. (1993). Biospecific haemosorbents based on proteinase inhibitor. I. Synthesis and properties. Biomaterials 14, 51–56. doi: 10.1016/0142-9612(93)90075-D
Polanowski, A., Cieslar, E., Otlewski, J., Nienartowicz, B., Wilimowska-Pelc, A., and Wilusz, T. (1987). Protein inhibitors of trypsin from the seeds of cucurbitaceae plants. Acta Biochim. Polan. 34, 395–406.
Prasad, E. R., Dutta-Gupta, A., and Padmasree, K. (2010a). Purification and characterization of a Bowman-Birk proteinase inhibitor from the seeds of black gram (Vigna mungo). Phytochemistry 71, 363–372. doi: 10.1016/j.phytochem.2009.11.006
Prasad, E. R., Merzendorfer, H., Madhurarekha, C., Dutta-Gupta, A., and Padmasree, K. (2010b). Bowman-Birk Proteinase Inhibitor from Cajanus cajan seeds: purification, characterization, and insecticidal properties. J. Agric. Food. Chem. 58, 2838–2847. doi: 10.1021/jf903675d
Prasain, J. K., and Barnes, S. (2007). Metabolism and bioavailability of flavonoids in chemoprevention: current analytical strategies and future prospectus. Mol. Pharm. 4, 846–864. doi: 10.1021/mp700116u
Pusztai, A. (1972). Metabolism of trypsin inhibitory proteins in the germinating seeds of kidney bean (Phaseolus vulgaris). Planta 107, 121–129. doi: 10.1007/BF00387718
Ragg, E. M., Galbusera, V., Scarafoni, A., Negri, A., Tedeschi, G., Consonni, A., et al. (2006). Inhibitory properties and solution structure of a potent Bowman-Birk protease inhibitor from lentil (Lens culinaris L.) seeds. FEBS J. 273, 4024–4039. doi: 10.1111/j.1742-4658.2006.05406.x
Rakashanda, S., Ishaq, M., Masood, A., and Amin, S. (2012). Antibacterial activity of a trypsin-chymotrypsin-elastase inhibitor isolated from Lavatera cashmeriana camb. seeds. J. Anim. Plant. Sci. 22, 983–986.
Rakashanda, S., Mubashir, S., Qureshi, Y., Hamid, A., Masood, A., and Amin, S. (2013b). Trypsin inhibitors from Lavatera cashmeriana camb. seeds: isolation, characterization and in-vitro cytoxic activity. Int. J. Pharm. Sci. Invent. 2, 55–65.
Rakashanda, S., Qazi, A. K., Majeed, R., Rafiq, S., Dar, I. M., Masood, A., et al. (2013a). Anti-proliferative Activity of Lavatera cashmeriana- Protease Inhibitors towards Human Cancer Cells. Asian. Pacific. J. Cancer. Prev. 14, 3975–3978. doi: 10.7314/APJCP.2013.14.6.3975
Richardson, M. J. (1991). “Seed storage proteins: the enzyme inhibitors,” in Methods in Plant Biochemistry, ed M. J. Richardson (New York, NY: Academic Press), 259–305.
Ryan, C. A. (1973). Proteolytic enzymes and their inhibitors in plants. Ann. Rev. Plant. Physiol. 24, 173–196. doi: 10.1146/annurev.pp.24.060173.001133
Ryan, C. A. (1990). Protease inhibitors in plants: genes for improving defences against insects and pathogens. Annu. Rev. Phytopathol. 28, 425–449. doi: 10.1146/annurev.py.28.090190.002233
Ryan, S. N., Liang, W. A., and McManus, M. T. (1998). A cysteine proteinase inhibitor purified from apple fruit. Phytochem. 49, 957–963. doi: 10.1016/S0031-9422(98)00206-4
Sakato, K., Tanaka, H., and Misawa, M. (1975). Broad specificity proteinase inhibitors in Scopolia japonica (solanaceae) cultured cells. Eur. J. Biochem. 55, 211–219. doi: 10.1111/j.1432-1033.1975.tb02153.x
Sakuta, C., Oda, A., Konishi, A., Yamakawa, S., Kamada, H., and Satoh, S. (2001). Cysteine proteinase gene expression in the endosperm of germinating carrot seeds. Biosci. Biotechnol. Biochem. 65, 2243–2248. doi: 10.1271/bbb.65.2243
Satheesh, L. S., and Murugan, K. (2011). Antimicrobial activity of protease inhibitor from leaves of Coccinia grandis (L.) Voigt. Indian J. Exp. Biol. 49, 366–374.
Sen, S., and Dutta, S. K. (2012). Evaluation of anti-cancer potential of ragi bifunctional inhibitor (RBI) from Elusine coracana on human chronic myeloid leukemia cells. Eur. J. Plant. Sci. Biotec. 6, 103–108.
Souza, Lda. C., Camargo, R., Demasi, M., Santana, J. M., and de Sá, C. M. (2014). Effects of an Anticarcinogenic Bowman-Birk Protease Inhibitor on Purified 20SProteasome and MCF-7 Breast Cancer Cells. PLoS ONE 9:e86600. doi: 10.1371/journal.pone.0086600
Sugano, M. (2006). Soy in Health and Disease Prevention. New York, NY: CRC Press, Taylor & Francis Group.
Sun, J., Wang, H., and Ng, T. B. (2010). Trypsin isoinhibitors with antiproliferative activity toward leukemia cells from phaseolus vulgaris cv “White Cloud Bean.” J. Biomed. Biotec. 2010:219793. doi: 10.1155/2010/219793
Sun, X. Y., Donald, S. P., and Phang, J. M. (2001). Testosterone and prostate specific antigen stimulate generation of reactive oxygen species in prostate cancer cells. Carcinogenesis 22, 1775–1780. doi: 10.1093/carcin/22.11.1775
Suzuki, A., Tsunogae, Y., Tanaka, I., Yamane, T., Ashida, T., Norioka, S., et al. (1987). The structure of Bowman-Birk type protease inhibitor A-II from peanut (Arachishypogaea) at 3.3 A resolution. J. Biochem. 101, 267–274.
Suzuki, K., Yano, T., Sadzuka, Y., Sugiyama, T., Seki, T., and Asano, R. (2005). Restoration of connexin 43 by Bowman-Birk protease inhibitor in M5067 bearing mice. Oncol. Rep. 13, 1247–1250.
Tamir, S., Bell, J., Finlay, T. H., Sakal, E., Smirnoff, P., Gaur, S., et al. (1996). Isolation, characterization and properties of a trypsin–chymotrypsin inhibitor from Amaranth seeds. J. Prot. Chem. 15, 219–229. doi: 10.1007/BF01887402
Tang, M., Asamoto, M., Ogawa, K., Naiki-Ito, A., Sato, S., Takahashi, S., et al. (2009). Induction of Apoptosis in the LNCaP Human Prostate Carcinoma Cell Line and Prostate Adenocarcinomas of SV40T Antigen Transgenic Rats by the Bowman-Birk. Pathol. Int. 59, 790–796. doi: 10.1111/j.1440-1827.2009.02445.x
Taussig, S. J., and Batkin, S. (1988). Bromelain, the enzyme complex of pineapple (Ananascomosus) and its clinical application: an update. Ethnopharmacology 22, 191–203. doi: 10.1016/0378-8741(88)90127-4
Tochi, B. N., Wang, Z., Xu, S. Y., and Zhang, W. (2008). Therapeutic application of pineapple protease (bromelain): a review. Pak. J. Nutr. 7, 513–520. doi: 10.3923/pjn.2008.513.520
Touil, T., Ciric, B., Ventura, E., Shindler, K. S., Gran, B., and Rostami, A. (2008). Bowman-Birk inhibitor suppresses autoimmune inflammation and neuronal loss in a mouse model of multiple sclerosis. J. Neurol. Sci. 271, 191–202. doi: 10.1016/j.jns.2008.04.030
Troncoso, M. F., Biron, V. A., Longhi, S. A., Retegui, L. A., and Wolfenstein-Todel, C. (2007). Peltophorum dubium and soybean Kunitz-type trypsin inhibitors induce human Jurkat cell apoptosis. Int. Immunol. Pharmacol. 7, 625–636. doi: 10.1016/j.intimp.2007.01.002
Tur Sinal, A., Birk, Y., Gertler, A., and Rigbi, M. (1972). A basic trypsin and chymotrypsin inhibitor from groundnuts (Arachis hypogaea). Biochim. Biophys. Acta 263, 666–672. doi: 10.1016/0005-2795(72)90048-7
Vogel, R., Trautschold, I., and Werle, E. (1968). Natural Proteinase Inhibitors. New York, NY: Academic Press.
Wan, X. S., Lu, L. J., Anderson, K. E., Ware, J. H., and Kennedy, A. R. (2000). Urinary excretion of Bowman-Birk inhibitor in humans after soy consumption as determined by a monoclonal antibody- based immunoassay. Cancer Epidemiol. Biomarkers Prev. 9, 741–747.
Wan, X. S., Meyskens, F. L. Jr., Armstrong, W. B., Taylor, T. H., and Kennedy, A. R. (1999a). Relationship between protease activity and neu oncogene expression in patients with oral leukoplakia Treated with the Bowman-Birk Inhibitor. Cancer Epidemiol. Biomarkers Prev. 8, 601–608.
Wan, X. S., Ware, J. H., Zhang, L., Newberne, P. M., Evans, S. M., Clark, L. C., et al. (1999b). Treatment with soybean-derived Bowman Birk inhibitor increases serum prostate-specific antigen concentration while suppressing growth of human prostate cancer xenografts in nude mice. Prostate. 41, 243–252. doi: 10.1002/(SICI)1097-0045(19991201)41:4<243::AID-PROS4>3.0.CO;2-F
Wang, H. X., and Ng, T. B. (2006). Concurrent isolation of a Kunitz-type trypsin inhibitor with antifungal activity and a novel lectin from Pseudostellaria heterophylla roots. BBRC 342, 349–353. doi: 10.1016/j.bbrc.2006.01.109
Ware, H. W., Wan, S., Newberne, P., and Kennedy, A. R. (1999). Bowman-Birk Concentrate Reduces Colon Inflammation in Mice with Dextran Sulphate Sodium-Induced Ulcerative Colitis. Dig. Dis. Sci. 44, 986–990. doi: 10.1023/A:1026616832119
Wattenberg, L. W. (1992). Inhibition of Carcinogenesis by Minor Dietary Constituents. Cancer Res. 52(Suppl.), 2085–2091.
Weder, J. K. P. (1981). “Protease inhibitors in the Leguminosae,” in Advances in Legume Systematics, Part I, Proceedings of. International Legume Conference, Vol 2, eds R. M. Polhill and P. H. Raven (Kew; England: Royal Botanic Gardens), 533–560.
Wieczorek, M., Otlewski, J., Cook, J., Parks, K., and Leluk, J. (1985). The squash family of serine proteinase inhibitors. Amino acid sequences and association equilibrium constants of inhibitors from squash, summer squash, zucchini, and cucumber seeds. Biochem. Biophys. Res. Commun. 126, 646–652. doi: 10.1016/0006-291X(85)90233-5
Yavelow, J., Collins, M., Birk, Y., Troll, W., and Kennedy, A. R. (1985). Nanomolar concentrations of Bowman-Birk soybean protease inhibitor suppress X-ray induced transformation in vitro. Proc. Natl. Acad. Sci. U.S.A. 82, 5395–5399. doi: 10.1073/pnas.82.16.5395
Keywords: BBI, cancer, food crops, plant protease inhibitors, pharmacological, therapeutics
Citation: Srikanth S and Chen Z (2016) Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy. Front. Pharmacol. 7:470. doi: 10.3389/fphar.2016.00470
Received: 18 April 2016; Accepted: 18 November 2016;
Published: 08 December 2016.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Ali Hussein Eid, American University of Beirut, LebanonLinghua Meng, Shanghai Institute of Materia Medica (CAS), China
Copyright © 2016 Srikanth and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Chen, zhong.chen@nie.edu.sg
 Sandhya Srikanth
Sandhya Srikanth Zhong Chen
Zhong Chen