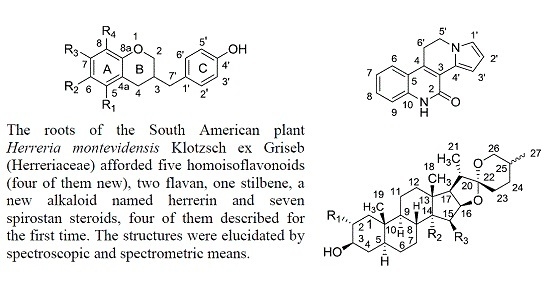
New Homoisoflavanes, a New Alkaloid and Spirostane Steroids from the Roots of Herreria montevidensis Klotzsch ex Griseb. (Herreriaceae) †
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Compound Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmeda-Hirschmann, G. Magic and medicinal plants of the Ayoreos of the Chaco Boreal. J. Ethnopharmacol. 1993, 39, 105–111. [Google Scholar] [CrossRef]
- Arenas, P. Etnobotánica Lengua-Maskoy; Fundación para la Educación, la Ciencia y la Cultura: Buenos Aires, Argentina, 1981; p. 149. [Google Scholar]
- Alvarez, J.M.; Lopes, R.C.; Bortolotto, I.M. The ethnobotany of Herreria montevidensis Klotzsch ex Griseb.-Herreriaceae, in Corumbá, Brazil. Econ. Bot. 2008, 62, 187–191. [Google Scholar] [CrossRef]
- Correia, P. Dicionário das Plantas úteis do Brasil; Ministerio da Agricultura e Instituto Brasileiro de Desenvolvimento Forestal: Rio de Janeiro, Brazil, 1984; Volume IV, p. 436, Volume VI, p. 21.
- Hegnauer, R. Chemotaxonomie der Pflanzen; Birkhäuser Verlag: Basel, Switzerland, 1963; Volume 2. [Google Scholar]
- Cecy, C.; Yassumoto, Y. Steroidal saponins in the roots of Herreria montevidensis (Liliaceae). Trib. Farm. 1973, 40, 52–55. [Google Scholar]
- Camarda, L.; Merlini, L.; Nasini, G. Dragon’s blood from Dracaena draco, structure of novel homoisoflavonoids. Heterocycles 1983, 20, 39–43. [Google Scholar]
- Masaoud, M.; Ripperger, H.; Porzel, A.; Adam, G. Flavonoids of Dragon’s Blood from Dracaena cinnabari. Phytochemistry 1995, 38, 745–749. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, H.; Xu, X.; Mei, W.; Dai, H. Antioxidant phenolic compounds of Dracaena cambodiana. Molecules 2010, 15, 8904–8914. [Google Scholar] [CrossRef] [PubMed]
- González, G.A.; León, F.; Sánchez-Pinto, L.; Padrón, J.I.; Bermejo, J. Phenolic compounds of Dragon’s Blood from Dracaena draco. J. Nat. Prod. 2000, 63, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Tinto, W.F.; Simmons-Boyce, J.L.; McLean, S.; Reynolds, W.F. Constituents of Agave americana and Agave barbadensis. Fitoterapia 2005, 76, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Miyamoto, T.; Linn, T.Z.; Li, F.; Win, N.N.; Tezuka, Y.; Esumi, H.; Kadota, S. Cytotoxic constituents of Soymida febrifuga from Myanmar. J. Nat. Prod. 2009, 72, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Makboul, M.A.; Attia, A.A.; Ali, D.T. Chromones and flavans from Pancratium maritimum. Phytochemistry 1990, 29, 625–627. [Google Scholar] [CrossRef]
- Liu, J.; Dai, H.-F.; Wu, J.; Zeng, Y.-B.; Mei, W.-L. Flavanes from Dracaena cambodiana. Z. Naturforsch. 2008, 63B, 1407–1410. [Google Scholar]
- Fernández, M.I.; Pedro, J.R.; Seoane, E. Two polyhydroxystilbenes from stems of Phoenix dactylifera. Phytochemistry 1983, 22, 2819–2821. [Google Scholar] [CrossRef]
- Buckingham, J. Dictionary of Natural Products on DVD, Version 25:1; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zhu, Y.; Zhang, P.; Yu, H.; Li, J.; Wang, M.-W.; Zhao, W. Anti-Helicobacter pylori and thrombin inhibitory components from Chinese Dragon’s Blood, Dracaena cochinchinensis. J. Nat. Prod. 2007, 70, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Biosynthesis of the 3-benzylchroman-4-one eucomin in Eucomis bicolor. Phytochemistry 1975, 14, 983–988. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Jain, D.C.; Gupta, R.K.; Thakur, R.S. Carbon-13 NMR spectroscopy of steroidal sapogenins and steroidal saponins. Phytochemistry 1985, 24, 2479–2496. [Google Scholar] [CrossRef]
- Brandão, M.G.L.; Zanetti, N.N.S.; Oliveira, P.; Grael, C.F.F.; Santos, A.C.P.; Monte-Mór, R.L.M. Brazilian medicinal plants described by 19th century European naturalists and in the Official Pharmacopoeia. J. Ethnopharmacol. 2008, 120, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.L.; Oliveira, V.B.; Viana, C.T.R.; Campos, P.P.; Silva, M.A.N.; Brandão, M.G.L. Antihyperlipidemic and antihyperglycemic effects of the Brazilian salsaparrilhas Smilax brasiliensis Spreng. (Smilacaceae) and Herreria salsaparrilha Mart. (Agavaceae) in mice treated with a high-refined-carbohydrate containing diet. Food Res. Int. 2015, 76, 366–372. [Google Scholar] [CrossRef]
- Jeon, S.-Y.; Kwon, S.-H.; Seong, Y.-H.; Bae, K.; Hur, J.-M.; Lee, Y.-Y.; Suh, D.-Y.; Song, K.-S. β-Secretase (BACE1)-inhibiting stilbenoids from Smilax Rhizoma. Phytomedicine 2007, 14, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Li, T.-X.; Ma, H.; Zhang, J.-F.; Jia, A.-Q. Tumoral cytotoxic and antioxidative phenylpropanoid glycosides in Smilax riparia A. DC. J. Ethnopharmacol. 2013, 149, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Wungsintaweekul, B.; Umehara, K.; Miyase, T.; Noguchi, H. Estrogenic and anti-estrogenic compounds from the Thai medicinal plant, Smilax corbularia (Smilacaceae). Phytochemistry 2011, 72, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Xiang, L.; Huang, W.; He, X. Spirostane saponins from Chinese onion (Allium chinense) exert pronounced anti-inflammatory and anti-proliferative activities. J. Funct. Foods 2016, 25, 208–219. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.

| Position | 1 CDCl3 + MeOH-d4 | 2 | 3 CDCl3 + MeOH-d4 | 4 CDCl3 + MeOH-d4 | 5 |
|---|---|---|---|---|---|
| 21 | 4.24 dd (10.4, 1.2) | 4.06 dd (10.4, 1.2) | 4.09 dd (10.4, 1.2) | 4.07 dd (10.4, 1.2) | 4.59 brs |
| 22 | 3.83 dd (10.4, 8.8) | 3.69 dd (10.4, 8.8) | 3.77 dd (10.4, 8.8) | 3.73 dd (10.4, 8.8) | - |
| 3 | 2.25 m | 2.15 m | 2.20 m | 2.17 m | - |
| 41 | 2.73 dd (16.0, 5.2) | 2.62 dd (16.0, 5.2) | 2.79 dd (16.0, 5.2) | 2.78 dd (16.0, 5.2) | 6.04 brs |
| 42 | 2.41 dd (16.0, 8.8) | 2.33 dd (16.0, 8.0) | 2.37 dd (16.0, 8.0) | 2.35 dd (16.0, 8.4) | |
| 5 | 6.63 d (8.4) | 6.75 d (8.4) | - | - | 6.53 d (8.0) |
| 6 | 6.48 d (8.4) | 6.28 dd (8.4, 2.5) | - | - | 6.44 d (8.0) |
| 8 | - | 6.21 d (2.5) | 6.18 s | 6.13 s | - |
| 2′,6′ | 7.04 d (7.6) | 6.95 d (8.0) | 7.04 d (8.0) | 7.02 d (8.0) | 7.01 d (8.0) |
| 3′,5′ | 6.78 d (7.6) | 6.70 d (8.0) | 6.75 d (8.0) | 6.77 d (8.0) | 6.73 d (8.0) |
| 7′1 | 2.58 dd (13.6, 7.6) | 2.52 dd (15.2, 7.6) | 2.69 dd (15.2, 7.6) | 2.61 dd (13.6, 7.2) | 3.28 brs |
| 7′2 | 2.52 dd (13.6, 7.2) | 2.45 dd (15.2, 7.6) | 2.59 dd (15.2, 7.6) | 2.56 dd (13.6, 7.2) | |
| Me | - | - | 2.06 s | 2.08 s | - |
| OMe | 3.84 s | 3.77 s | 3.66 s | - | 3.81 s |
| OMe | - | - | 3.75 s | 3.67 s | - |
| Position | 1 CDCl3 + MeOH-d4 | 2 | 3 CDCl3 + MeOH-d4 | 4 CDCl3 + MeOH-d4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 2 | 70.0 t | 69.9 t | 69.6 t | 69.6 t | 68.0 t | 77.1 d | 77.0 d |
| 3 | 34.0 d | 34.3 d | 33.9 d | 33.9 d | 128.6 s | 19.4 t | 19.5 t |
| 4 | 30.3 t | 30.2 t | 25.5 t | 25.5 t | 119.6 d | 29.4 t | 29.5 t |
| 4 a | 114.4 s | 112.8 s | 111.4 s | 110.0 s | 116.6 s | 103.9 s | 106.2 s |
| 5 | 124.4 d | 124.4 d | 157.3 s * | 157.2 s | 121.1 d | 155.7 s | 155.7 s |
| 6 | 107.5 d | 108.1 d | 107.0 s | 106.5 s | 107.6 d | 90.9 d | 87.8 d |
| 7 | 147.5 s | 150.0 s | 154.0 s | 154.3 s | 149.0 s | 154.1 s | 155.0 s |
| 8 | 134.8 s | 102.8 d | 95.3 d | 98.7 d | 134.8 s | 102.6 s | 103.5 s |
| 8a | 147.2 s | 154.8 s | 157.2 s * | 152.9 s | 145.2 s | 152.9 s | 155.0 s |
| 1′ | 130.4 s | 130.3 s | 131.6 s | 130.5 s | 131.0 s | 133.5 s | 134.4 s |
| 2′, 6′ | 129.9 d | 129.9 d | 130.1 d | 129.8 d | 129.8 d | 127.1 d | 127.2 d |
| 4‘ | 154.8 s | 154.9 s | 154.3 s | 154.9 s | 155.2 s | 156.0 s | 156.6 s |
| 3′, 5′ | 115.2 d | 115.2 d | 115.2 d | 115.1 d | 115.2 d | 115.1 d | 115.1 d |
| 7′ | 36.9 t | 37.0 t | 37.2 t | 37.2 t | 38.8 t | - | - |
| CH3 | - | - | 8.5 q | 8.2 q | - | 7.6 q | 7.8 q |
| 5-OCH3 | - | - | 59.9 q | 59.7 q | - | - | 56.0 q ** |
| 7-OCH3 | - | 60.7 q | 55.5 q | - | - | 55.3 q | 55.5 q ** |
| 8-OCH3 | 60.7 q | - | - | - | 60.8 q | - | - |
| Position | δC, Type | H | δH (J in Hz) | HMBC | NOE |
|---|---|---|---|---|---|
| 2 | 172.8 C | ||||
| 3 | 132.7 C | ||||
| 4 | 121.0 C | ||||
| 5 | 127.2 C | ||||
| 6 | 120.6 CH | 6 | 7.66 d (8) | 4 (w), 5 (w), 8 (s), 10 (s) | 6′(3) |
| 7 | 120.3 CH | 7 | 7.17 ddd (8, 8, 1) | 5 (s), 9 (s) | |
| 8 | 126.3 CH | 8 | 7.36 ddd (8, 8, 1) | 6 (s), 10 (s) | |
| 9 | 112.3 CH | 9 | 7.46 d (8) | 5 (s), 7 (s) | N-H(3) |
| 10 | 136.6 C | ||||
| 1′ | 119.9 CH | 1′ | 7.34 dd (4, 2) | 3′ (m), 4′ (m) | |
| 2′ | 109.3 CH | 2′ | 6.26 dd (4, 2) | 1′ (w), 3′ (s), 4′ (s) | |
| 3′ | 129.6 CH | 3′ | 6.90 dd (2, 2) | 1′ (s), 2′ (s), 5′ (w), 4′ (s) | 5´(3) |
| 4′ | 133.9 C | - | |||
| 5′ | 50.4 CH2 | 5′ | 4.48 m | 3′ (m), 6′ (m), 4′ (m), 4 (s) | 3′(5) |
| 6′ | 25.4 CH2 | 6′ | 3.40 m | 3 (s), 4 (s), 5 (s), 5′ (m) | 6 (5) |
| NH | 9.45 br s | 5 (w) | 9 (5) |
| Position | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|
| 2 | 5.02 ddd (12, 10, 5) | 5.03 ddd (12, 10, 5) | 5.04 ddd (12, 10, 5) | 5.02 ddd (12, 10, 5) | ‡ | 5.03 ddd (12, 10, 5) | 5.10 ddd (12, 10, 5) |
| 3 | 4.78 ddd (11, 10, 5) | 4.79 ddd (11, 10, 5) | 4.80 ddd (11, 10, 5) | 4.78 ddd (11, 10, 5) | 4.67 dddd (11, 11, 4, 4) | 4.79 ddd (11, 10, 5) | 4.72 ddd (11, 10, 5) |
| 6 | * | * | * | * | * | * | 5.47 br ddd (5,2,2) |
| 15 α | 1.65 m | 1.94 dd (13, 8) | 4.10 ddd (5, 3, 2) | 1.65 m | 1.94 dd (13, 8) | 1.94 dd (13, 8) | ‡ |
| 15 β | 1.20 m | 1.59 dd (13, 6) | 15-OH, 2.23 brs | 1.20 m | 1.58 dd (13, 6) | 1.59 dd (13, 6) | ‡ |
| 16 | 4.37 ddd (8, 8, 6) | 4.62 ddd (8, 8, 6) | 4.34 dd (8, 5) | 4.37 ddd (8, 8, 6) | 4.63 ddd (8, 8, 6) | 4.62 ddd (8, 8, 6) | 4.62 ddd (8, 8, 6) |
| 17 | 1.75 dd (8, 7) | 2.31 dd (8, 7) | 1.94 dd (8, 7) | 1.75 dd (8, 7) | 2.31 dd (8, 7) | 2.31 dd (8, 7) | 2.32 dd (8, 7) |
| 18 | 0.73 s | 0.90 s | 0.99 s | 0.73 s | 0.86 s | 0.90 s | 0.93 s |
| 19 | 0.90 s | 0.95 s | 0.95 s | 0.90 s | 0.91 s | 0.95 s | 1.15 s |
| 20 | 1.85 m | 1.90 m | ‡ | 1.80 m | 1.86 m | 1.86 m | ‡ |
| 21 | 0.93 d (7) | 0.97 d (7) | 0.96 d (7) | 0.96 d (7) | 1.00 d (7) | 1.00 d (7) | 0.99 d (7) |
| 26 α | 3.34 dd (11, 11) | 3.36 dd (11, 11) | 3.38 dd (11, 11) | 3.27 brd (11) | 3.30 brd (11) | 3.30 brd (11) | 3.36 dd (11, 11) |
| 26 β | 3.45 ddd (11, 5, 2) | 3.48 ddd (11, 5, 2) | 3.51 ddd (11, 5, 2) | 3.92 dd (11, 3) | 3.93 dd (11, 3) | 3.93 dd (11, 3) | 3.48 ddd (11, 5, 2) |
| 27 | 0.76 d (7) | 0.79 d (7) | 0.80 d (7) | 1.05 d (7) | 1.08 d (7) | 1.08 d (7) | 0.79 d (7) |
| OAc | 2.01 s | 2.01 s | 2.01 s | 2.01 s | 2.01 s | 2.01 s | 2.01 s |
| 2.00 s | 2.01 s | 2.00 s | 2.00 s | - | 2.01 s | 2.00 s |
| Position | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|
| 1 | 42.1 t | 42.4 t | 42.1 t | 42.3 t | 36.8 t | 42.3 t | 42.3 t |
| 2 | 71.6 d | 71.8 d | 71.8 d | 71.9 d | 28.3 t | 71.8 d | 74.3 d |
| 3 | 74.4 d | 74.5 d | 74.5 d | 74.6 d | 73.5 d | 74.5 d | 71.4 d |
| 4 | 32.5 t | 32.8 t | 32.6 t | 32.7 t | 33.9 t | 32.7 t | 36.2 t |
| 5 | 43.9 d | 43.9 d | 44.2 d | 44.1 d | 44.3 d | 43.8 d | 137.1 s |
| 6 | 27.4 t | 27.4 t | 27.4 t | 27.5 t | 27.4 t | 27.4 t | 123.4 d |
| 7 | 31.5 t | 26.7 t | 31.1 t | 31.6 t | 26.9 t | 26.7 t | 26.0 t |
| 8 | 34.2 d | 37.8 d | 30.5 d | 34.3 d | 38.4 d | 37.7 d | 34.3 d |
| 9 | 53.8 d | 46.6 d | 54.5 d | 54.0 d | 46.7 d | 46.6 d | 43.0 d |
| 10 | 36.9 s | 37.3 s | 37.2 s | 37.1 s | 35.8 s | 37.3 s | 38.3 s |
| 11 | 21.0 t | 20.1 t | 21.3 t | 21.1 t | 20.0 t | 20.1 t | 19.7 t |
| 12 | 39.6 t | 31.8 t | 42.1 t | 39.8 t | 31.9 t | 31.8 t | 31.4 t |
| 13 | 40.3 s | 44.5 s | 40.6 s | 40.4 s | 44.5 s | 44.5 s | 44.3 s |
| 14 | 55.8 d | 87.9 s | 60.2 d | 56.0 d | 88.1 s | 87.9 s | 87.1 s |
| 15 | 31.7 t | 39.5 t | 69.6 d | 31.8 t | 39.3 t | 39.4 t | 39.3 s |
| 16 | 80.6 d | 80.8 d | 82.0 d | 80.8 d | 81.0 d | 80.9 d | 80.7 d |
| 17 | 61.7 d | 58.7 d | 61.1 d | 61.9 d | 58.5 d | 58.5 d | 58.8 d |
| 18 | 16.3 q | 20.1 q | 19.0 q | 16.4 q | 20.2 q | 20.1 q | 19.8 q |
| 19 | 12.8 q | 12.8 q | 12.8 q | 12.9 q | 12.1 q | 12.8 q | 14.7 q |
| 20 | 41.4 d | 41.6 d | 42.4 s | 42.1 d | 42.1 d | 42.1 d | 41.6 d |
| 21 | 14.2 q | 14.7 q | 14.2 q | 14.3 q | 14.5 q | 14.5 q | 14.7 q |
| 22 | 109.0 s | 109.5 s | 110.0 s | 109.6 s | 109.9 s | 109.9 s | 109.5 s |
| 23 | 31.2 t | 31.5 t | 31.1 t | 25.7 t | 25.8 t | 25.8 t | 31.4 t |
| 24 | 28.6 t | 28.8 t | 28.5 t | 25.9 t | 26.0 t | 26.0 t | 28.8 t |
| 25 | 30.1 d | 30.2 d | 30.1 d | 27.0 d | 27.0 d | 27.0 d | 30.2 d |
| 26 | 66.6 t | 66.8 t | 67.1 t | 65.1 t | 65.1 t | 65.1 t | 66.8 t |
| 27 | 17.0 q | 17.1 q | 17.0 q | 16.0 q | 16.1 q | 16.1 q | 17.1 d |
| OAc | 170.3 s | 170.6 s | 170.5 s | 170.6 s | 170.7 s | 170.6 s | 170.5 s |
| 21.0 q | 21.1 q | 21.1 q | 21.1 q | 21.5 q | 21.1 q | 21.2 q | |
| OAc | 170.2 s | 170.6 s | 170.4 s | 170.5 s | - | 170.6 s | 170.4 s |
| 21.0 q | 21.2 q | 21.1 q | 21.1 q | - | 21.2 q | 21.1 q |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutra-Behrens, M.; Schmeda-Hirschmann, G. New Homoisoflavanes, a New Alkaloid and Spirostane Steroids from the Roots of Herreria montevidensis Klotzsch ex Griseb. (Herreriaceae). Molecules 2016, 21, 1589. https://doi.org/10.3390/molecules21111589
Dutra-Behrens M, Schmeda-Hirschmann G. New Homoisoflavanes, a New Alkaloid and Spirostane Steroids from the Roots of Herreria montevidensis Klotzsch ex Griseb. (Herreriaceae). Molecules. 2016; 21(11):1589. https://doi.org/10.3390/molecules21111589
Chicago/Turabian StyleDutra-Behrens, María, and Guillermo Schmeda-Hirschmann. 2016. "New Homoisoflavanes, a New Alkaloid and Spirostane Steroids from the Roots of Herreria montevidensis Klotzsch ex Griseb. (Herreriaceae)" Molecules 21, no. 11: 1589. https://doi.org/10.3390/molecules21111589