PIRS: Development of Multiple Anticalin® Products Continues…
By David Bautz, PhD
NASDAQ:PIRS
Business Update
Pieris Pharmaceuticals (PIRS) is developing the Anticalin® class of biotherapeutic compounds, for which they have exclusive worldwide rights. Anticalins® are a new class of novel, engineered proteins that are based on lipocalins, which are naturally-occurring proteins with variable loop regions supported by a rigid framework that bind a wide array of compounds (just like antibodies). Pieris scientists have developed a means of modifying the lipocalins to confer unique binding specificities against virtually any biological target, and dubbed the new compounds ‘Anticalins®’ (antibody + lipocalin).
The company is increasingly focusing on the application of Anticalins® in immunooncology (IO), as the natural strengths of the platform are well-suited to that area. In addition, the company continues development of Anticalins® for diseases of high unmet need, including iron deficiency in patients with chronic kidney disease and uncontrolled asthma. Pieris has entered into a number of partnerships with companies such as Roche, Daiichi Sankyo, and Sanofi. These partnerships are an important source of cash to fund the company’s pipeline products, but perhaps more importantly, serve as a validation for the Anticalin® platform.
Anticalins® in Immunooncology
The PRS-300 series is focused on IO products that utilize different combinations of monoclonal antibodies and Anticalins® as a means of differentiation from other IO products currently available and under development. In addition, Pieris is collaborating with Roche on an IO product that is independent of Pieris’ developing IO pipeline.
PRS-343
PRS-343 is a bispecific compound that contains a CD137-specific Anticalin® genetically linked to a HER2-specific monoclonal antibody (trastuzumab), and is the lead compound from the company’s IO program. CD137 is a tumor necrosis factor receptor (TNFR) family member with costimulatory function that regulates T-cell proliferation and survival and is expressed on activated CD8+ and CD4+ T-cells (Watts, 2005). Since CD8+ T-cells play a pivotal role in tumor immunity, enhancing CD137 costimulation could promote the generation of protective antitumor immune responses.
Pieris presented the first in vivo data for PRS-343 at the 2016 American Association of Cancer Research (AACR) annual meeting. To test the efficacy of PRS-343, Pieris utilized NOG mice, which are immunocompromised and allow for the engraftment of human cells that can then proliferate and differentiate (Ito et al., 2002). The mice were engrafted with SKOV-3 cells, a human ovarian cancer cell line, which highly expresses HER2. Once the SKOV-3 tumors reached approximately 120 mm3, the mice were engrafted with human peripheral blood mononuclear cells (PBMC; to act as a surrogate immune system) and treatment began one hour later. Groups of ten mice were treated with placebo, isotype control antibody, PRS-343 (20 μg), PRS-343 (100 μg), or anti-CD137 antibody for a total of three treatments each seven days apart (Days 0, 7, 14). The following figure shows the tumor growth for the five groups of mice.

The data show that PRS-343 at both doses inhibits tumor growth, and even causes tumor regression for the first two weeks at the 100 μg dose. This is in stark contrast to the placebo treated mice that exhibited almost linear tumor growth for the three weeks of the experiment. The anti-CD137 antibody appeared to have some effect on tumor growth, however it’s difficult to determine how much since those mice faired very similarly to the isotype control-treated mice.
Perhaps the most interesting data to be reported by Pieris in this presentation was the toxicity data concerning the anti-CD137-treated animals. The following figures show the percentage of CD45+ cells amongst the PBMC cell population on Day 20 of the experiment. Mice treated with anti-CD137 antibody had a very high percentage of CD45+ cells (left panel), with almost 50% of those cells being CD8+ T-cells (middle panel). The net result of this was that a very high percentage of the anti-CD137-treated mice developed graft versus host disease and 50% of them died spontaneously or had to be sacrificed prior to Day 20 (right panel).
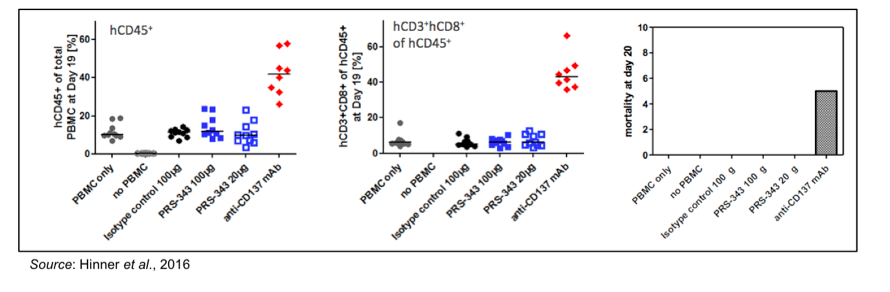
These results are very important in that they show peripheral activation of anti-CD137 can lead to toxic side effects, which was seen in a Phase 1 study of an anti-CD137 antibody that had to be discontinued due to toxicity (Yao et al., 2013). PRS-343 does NOT cause activation or expansion of peripheral T-cells, thus from a safety standpoint these data allude to the fact that PRS-343 may have a cleaner safety profile than previously tested anti-CD137 monoclonal antibodies. This exemplifies another advantage of the Anticalin® platform, which is the ability to target certain antigens in a unique manner that would otherwise be more difficult with a traditional monoclonal antibody approach.
The company will present additional data sets at scientific conferences in the second half of 2016 and IND-enabling studies are continuing such that a Phase 1 multiple ascending dose study in HER2-positive cancer patients can be initiated in the second half of 2017. In that study, the company is planning to target high unmet medical need cancers, which includes HER2-positive gastrointestinal cancers, muscle invasive bladder cancer, and HER2-positive metastatic breast cancer.
PRS-332
The newest addition to the company’s pipeline is PRS-332, which is a multi-immune checkpoint blockade molecule composed of an anti-PD-1 antibody genetically linked to an Anticalin® against an undisclosed checkpoint protein. PD-1 (Programmed cell death protein 1) is an immunoinhibitory receptor that is expressed on T-cells, B-cells, monocytes, and natural killer cells. Binding with its ligands, PD-L1 and PD-L2, causes suppression of the immune response. Many tumors express PD-L1, which results in an inability for the immune system to attack the tumor. Inhibiting this interaction can lead to immune system activation and a subsequent antitumor response.
A number of biopharmaceutical companies are developing or have developed antibodies that target the PD-1/PD-L1 axis. The anti-PD-1 antibodies nivolumab (Opdivo®) and pembrolizumab (Keytruda®) and the anti-PD-L1 antibody atezolizumab (Tecentriq®) have all been approved by the FDA for various types of cancer. While early results with these antibodies have been encouraging, particularly in certain types of cancers such as melanoma and kidney cancer, a robust response appears to be limited to a subset of treated patients, and it is becoming more evident that combination therapies are going to be necessary to increase the overall response rates in most types of cancer.
Pieris recently entered into a license and transfer agreement with Enumeral Biomedical Holdings, Inc. (ENUM) pursuant to Enumeral’s anti-PD-1 antibody program ENUM 388D4 for its potential use as a multispecific protein containing an Anticalin® protein. Pieris paid Enumeral a $1 million license fee and total development milestones of $37.8 million are possible along with net sales milestone payments of up to $67.5 million. Enumeral will also receive low-to-lower middle single digit royalty on net sales.
Preclinical development has initiated on at least one anti-checkpoint protein Anticalin® that will be combined with the anti-PD-1 antibody. The company has built various permutations of the bi-specific compound and hopes to have a development candidate selected by the end of 2016. The long-term vision for this program is to eventually test it in combination with PRS-343, assuming positive data is seen with both compounds as a monotherapy.
Anticalins® in Anemia and Asthma
PRS-080
PRS-080 is an Anticalin® that targets hepcidin, a 25 amino acid peptide produced in the liver and excreted into the bloodstream where it binds to ferroportin, an iron transporter found on duodenal enterocytes, reticuloendothelial macrophages, and hepatocytes (Donovan et al., 2006). Binding of hepcidin to ferroportin induces its internalization and degradation, resulting in iron accumulation in enterocytes, macrophages, and the liver. Hepcidin expression is induced by inflammatory cytokines, which is hypothesized to result in the sequestration of iron from invading pathogens (Babitt et al., 2010). This increased hepcidin expression results in many of the hallmarks of chronic diseases, including iron sequestration, hypoferremia, and anemia.
Pieris is developing PRS-080 as a treatment for functional iron deficiency (FID) in anemic patients with chronic kidney disease (CKD), which is a progressive loss of kidney function over a period of months or years. FID in CKD patients is characterized by low circulating iron levels that limits erythropoiesis even when these patients have sufficient iron stores in their body. These patients have low serum transferrin (a measure of circulating iron) and normal or high serum ferritin (a marker of body iron stores). FID is characteristic of a chronic inflammatory state, which in CKD patients may be due to an increased incidence of infections and/or the induction of inflammatory cytokines by the hemodialysis procedure.
The company presented results at the American Society of Hematology (ASH) 2015 annual meeting from a placebo controlled, double blind, Phase 1 study, which consisted of six cohorts of healthy volunteers treated with ascending doses of PRS-080 in of 0.08, 0.4, 1.2, 4.0, 8.0, and 16.0 mg/kg (NCT02340572). Six patients in each cohort received drug while two patients received placebo through a two hour intravenous infusion. A total of 39 adverse events (AEs) were reported during or after treatment in 22 subjects. All AEs were mild or moderate and there were no serious AEs reported. Importantly, there were no infusion reactions or hypersensitivities noted during the study. In addition, no anti-drug antibodies were detected from participants treated with PRS-080.
A decrease in free hepcidin was noted beginning one hour after administration of PRS-080 along with a transient increase in serum iron concentration and transferrin saturation. Serum iron concentrations reached approximately 50 μM in individual subjects but did not increase further with increasing dose. The normal range for serum iron concentration is approximately 11-30 μM. We believe this is a very important data point to come out of this trial, because if PRS-080 is able to increase normal iron levels to above normal, it stands to reason that it should be able to increase anemic patients iron levels to within the normal range.
The company is currently conducting a single ascending dose Phase 1b clinical trial of PRS-080 in hemodyalisis-dependent patients with end-stage renal disease (ESRD), with results from this first-in-patient study expected by the end of 2016. Following that trial, the company will move quickly into a multiple ascending dose trial in order to evaluate the effects on hemoglobin levels. We believe results from the multi-dose trial will be reported in mid-2017. We now anticipate the company continuing later stage development of PRS-080 through a partnership, particularly since the company seems to be devoting more resources to the IO pipeline. With approximately 180,000 dialysis patients worldwide having functional iron deficiency, we estimate PRS-080 could have greater than $1 billion in peak sales.
PRS-060
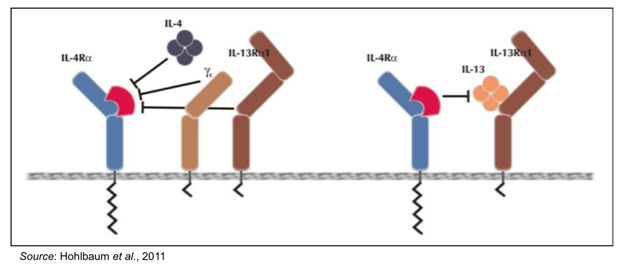
PRS-060 is an Anticalin® that has very high affinity for the alpha subunit of the IL-4 receptor (IL-4Ra; Kd ~ 20 pM) and does not bind to the related cytokine receptors IL-6R, IL-18R, and IL-23R. By targeting IL-4Ra, PRS-060 inhibits binding of both IL-4 and IL-13, as depicted in the following graphic.
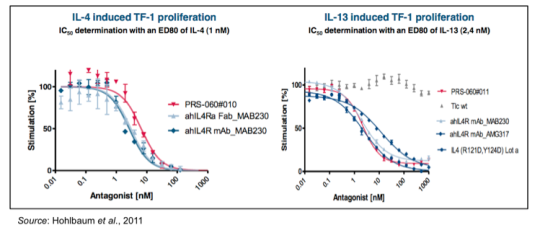
The inhibition of IL-4 and IL-13 binding by PRS-060 was shown in a series of preclinical experiments involving erythroleukemic (TF-1) cells that proliferate upon stimulation with a wide range of cytokines. The cells were preincubated for 30 min with PRS-060 or other IL-4Ra antagonists prior to stimulation with IL-4 (left) or IL-13 (right). Cell proliferation was measured five days after treatment and graphed as a function of increasing amounts of IL4Ra antagonist. The results show that PRS-060 is able to inhibit both IL-4 and IL-13 dependent proliferation.
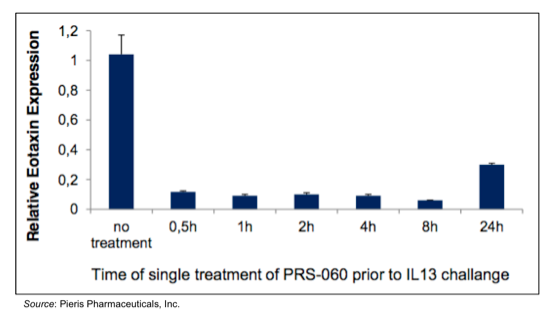
Pieris has formulated PRS-060 for pulmonary delivery by inhalation, which further shows the flexibility of the Anticalin® platform as monoclonal antibodies are currently unable to be dosed through inhalation. Efficacy of inhaled PRS-060 is shown in the following graphic, in which eotaxin expression (a marker of airway inflammation) was induced by administration of IL-13 to the lungs of mice after pre-treatment with inhaled PRS-060. The data shows that eotaxin expression is inhibited for up to 24 hours following a single inhaled dose of PRS-060.
IL-4Ra is a clinically validated target for the treatment of asthma, as shown by clinical trial results with the IL-4Ra specific monoclonal antibody dupilumab, which is being developed by Regneron Pharmaceuticals (REGN) and Sanofi. The results from a Phase 2a study showed that treatment with dupilumab resulted in a significant decrease in asthma exacerbations and an improvement in the ACQ5 score that was maintained through 12 weeks of treatment (Wenzel et al., 2013). In April 2016, the results of a pivotal Phase 2b study of dupilumab in adults with asthma were published in The Lancet (Wenzel et al., 2016). The results showed the study met its primary endpoint of improving lung function in both unselected severe asthma patients and those with “allergic” asthma. Dupilumab is administered subcutaneously, and in both studies the most common adverse events were injection-site reaction, nasopharyngitis, and upper respiratory tract infection. While dupliumab has shown clinical efficacy in treating asthma, we believe administering PRS-060 through inhalation could offer a number of potential advantages, including lower systemic side effects, a smaller dose, and a more convenient dosing method.
Currently, PRS-060 is still in preclinical development with the final IND enabling studies being conducted in 2016 in preparation for a first-in-man study to be initiated in the first half of 2017. With well over 1 million potential patients worldwide, PRS-060 has blockbuster potential.
Financial Update
On August 11, 2016, Pieris announced financial results for the second quarter of 2016. The company recognized $1.1 million in revenue, which was comprised of $0.7 million recognized from the $6.5 million upfront payment from Roche for the IO collaboration and $0.4 million for R&D services provided to Roche in relation to the IO collaboration. Net loss for the second quarter was $5.9 million, or $0.14 per share, and included $4.5 million in R&D expenses and $2.4 million in G&A expenses. Pieris exited the second quarter of 2016 with $40.4 million in cash and cash equivalents, compared to $29.3 million as of Dec. 31, 2015. This increase in cash was the result of a $16.5 million private placement, where the company sold 8,188,804 units at a price of $2.015 per unit. Each unit consisted of one share of Pieris’ common stock, 0.4 warrants to purchase one share of common stock at an exercise price of $2.00 per share, and 0.2 warrants to purchase one share of common stock at an exercise price of $3.00 per share. The warrants are exercisable for a period of five years from the date of issuance. We estimate the company now has sufficient capital to fund operations well into 2018.
Conclusion and Valuation
The Anticalin® platform is unrivaled at this moment in terms of both its flexibility and adaptability. With a near 100% success rate in identifying Anticalin’s® that bind to a target of choice, along with the ability to combine Anticalin’s® together in a virtually limitless combination, the full capability of the platform is only limited by the imagination of those using it. In addition, the company’s focus on IO is the right move, as real success with IO treatments will necessitate a multi-target approach, something for which the Anticalin® platform is particularly well-suited. This is especially true for the PD-1/PD-L1 signaling axis, as that market is becoming quite crowded. However, with the ability to pair up an anti-PD-1 antibody with an Anticalin(s)® targeting other checkpoint proteins, Pieris will be able to offer completely differentiated multi-specific PD-1 compounds, something that should lead to success in the clinic and pique the interest of potential partners.
We view the Anticalin® platform as a transformative technology that will lead to novel treatments in a variety of disease areas. Our probability adjusted discounted cash flow model leads to a valuation of $8 per share, and we believe any investor interested in small cap biotech should take a serious look at Pieris.
READ THE FULL RESEARCH REPORT HERE
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR and to view our disclaimer.
