May 23, 2016 report
Benzoquinone additive promotes solution-phase discharge in lithium-air batteries
(Phys.org)—Lithium-ion batteries are a mainstay in electronic devices because they have a long lifespan and can discharge at high voltages. But lithium-ion batteries still have some limitations, leading scientists to explore other possibilities involving lithium. One enticing possibility is the lithium-air battery. The practical lithium-air battery's energy storage is predicted to be three-to-four-fold higher than that of today's lithium-ion batteries. This high energy storage makes it an excellent candidate for powering electric cars.
However, lithium-air batteries have several hurdles to overcome before they can become practically useful. In order to make a stable Li-O2 battery, the electrolyte solution needs to be a low-polarity (weakly solvating) solvent. Unfortunately, using such solvents also limits the battery performance by unacceptable levels due to product formation on the cathode surface. Researchers from the University of Oxford have added DBBQ (2,5-di-tert-butyl-1,4-benzoquinone) to a weakly solvating electrolyte solution, LiTFSI in ether, and found that not only did this electrolyte solution prevent deposits on the cathode surface, leading to a much higher energy storage (80-to-100-fold increase), but it also increased the reaction rate. Their work appears in Nature Materials.
When Li-O2 batteries are discharged, O2 at the cathode is reduced to Li2O2, which can grow on the cathode surface, usually a porous carbon fiber, creating a film that can eventually hinder further use of the battery. If Li2O2 could form in solution rather on the cathode surface, this would overcome this problem. However, solvents that would allow for this (i.e., polar or strongly solvating) are vulnerable to reacting with oxygen anions and forming unwanted side products, diminishing the battery's lifetime and capacity.
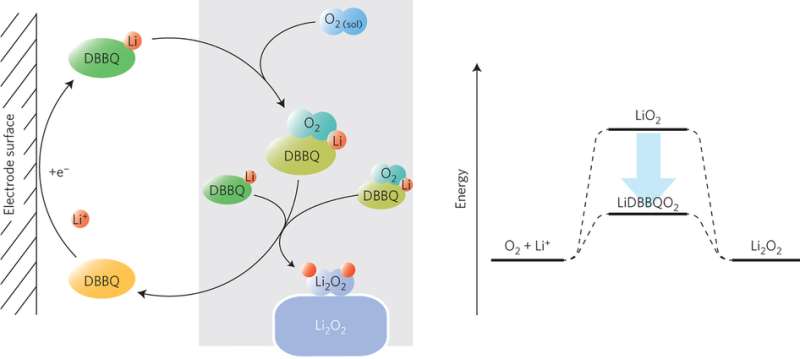
In an effort to work with a weakly polar solvent and keep lithium oxide products in solution, Xiangwen Gao, Yuhui Chen, Lee Johnson, and Peter G. Bruce looked at the Quinone family because their electrochemistry is in the right range for oxygen reduction and prior research has shown that they may serve as solution catalysts. As it turns out, DBBQ enhances oxygen reduction, but it serves as more than an electrocatalyst or an electron shuttle. The addition of DBBQ creates a completely different reaction mechanism from the typical mechanism in a Li-O2 cell.
The oxidation-reduction reaction of lithium metal and oxygen proceeds through a lithium oxide intermediate, LiO2. The reaction's energetics as well as many of the reaction's obstacles hinge on this intermediate. This is a high-energy intermediate, thus requiring a high thermodynamic overpotential, which lowers the battery voltage. It is also soluble in polar solvents. Its solubility determines whether Li2O2 forms in solution or on the cathode surface. These intermediate oxides and polar solvents lead to unwanted side reactions.
The addition of DBBQ involves a completely different mechanism that bypasses the LiO2 intermediate. Instead, DBBQ is reduced at the electrode surface, forming a lithium-DBBQ complex. In the presence of DBBQ, O2 reduction occurs at the same reduction potential for DBBQ, therefore at a higher potential (lower overpotential) than the reduction of O2 through the LiO2 intermediate. This is due to the formation of a LiDBBQO2 intermediate complex.
The LiDBBQO2 intermediate reacts with another LiDBBQ complex to form Li2O2 in solution, which was confirmed using a TiOSO4 titration test. The composition of the lithium oxide product was confirmed using powder x-ray diffraction, infrared and Raman spectroscopy, and in situ differential electrochemical mass spectroscopy, which showed Li2O2 was predominantly formed. SEM studies confirmed that Li2O2 particles were formed from solution rather than at the cathode surface.
The addition of DBBQ to a weakly solvating electrolyte results in a completely different reaction mechanism that involves the reduction of O2 via a LiDBBQ complex. This mechanism results in the formation of Li2O2 product in solution. By eliminating the LiO2 intermediate, Gao, et al. eliminated the source of cathode deposits, and were able to increase the cell's capacity by 80-to-100-fold. Their mechanism results in higher current densities while halving the overpotential.
This study provides important insights that will help make lithium-air batteries a practical reality.
More information: Xiangwen Gao et al. Promoting solution phase discharge in Li–O2 batteries containing weakly solvating electrolyte solutions, Nature Materials (2016). DOI: 10.1038/nmat4629
Abstract
On discharge, the Li–O2 battery can form a Li2O2 film on the cathode surface, leading to low capacities, low rates and early cell death, or it can form Li2O2 particles in solution, leading to high capacities at relatively high rates and avoiding early cell death. Achieving discharge in solution is important and may be encouraged by the use of high donor or acceptor number solvents or salts that dissolve the LiO2 intermediate involved in the formation of Li2O2. However, the characteristics that make high donor or acceptor number solvents good (for example, high polarity) result in them being unstable towards LiO2 or Li2O2. Here we demonstrate that introduction of the additive 2,5-di-tert-butyl-1,4-benzoquinone (DBBQ) promotes solution phase formation of Li2O2 in low-polarity and weakly solvating electrolyte solutions. Importantly, it does so while simultaneously suppressing direct reduction to Li2O2 on the cathode surface, which would otherwise lead to Li2O2 film growth and premature cell death. It also halves the overpotential during discharge, increases the capacity 80- to 100-fold and enables rates >1 mA cmareal−2 for cathodes with capacities of >4 mAh cmareal−2. The DBBQ additive operates by a new mechanism that avoids the reactive LiO2 intermediate in solution.
Journal information: Nature Materials
© 2016 Phys.org