In a case of serendipity, researchers at the University of Texas at Arlington started out trying to develop new security-related radiation detection and stumbled upon a potential breakthrough in cancer treatment.
In the research, which will be published in the August edition of the Journal of Biomedical Nanotechnology (“A New X-Ray Activated Nanoparticle Photosensitizer for Cancer Treatment”), Wei Chen, professor of physics at the UT Arlington, was exposing copper-cysteamine (Cu-Cy) nanoparticles to X-rays when he noticed an unusual luminescence over a time-lapse exposure. When he investigated the cause of the Cu-Cy nanoparticle luminescence, he realized that the particles were losing energy as they emitted singlet oxygen, a toxic byproduct that also happens to be used to in photodynamic cancer therapy to damage cancer cells.
“This new idea is simpler and better than previous photodynamic therapy methods. You don’t need as many steps. This material alone can do the job,” Chen, who is also leading federally-funded cancer research, said in a press release. “It is the most promising thing we have found in these cancer studies and we’ve been looking at this for a long time.”
Photodynamic therapy (PDT) is a technique in which some photosensitizer is introduced into tumor tissue. When exposed to light, the photosensizer produces singlet oxygen that kills the cancer cells. Two years ago, this blog covered research from Rice University related to a similar PDT technique in which gold nanoparticles were introduced near a tumor and then subjected to light. This light exposure to the cluster of nanoparticles created bubbles that burst, temporarily ripping open small pores in the cell membranes that allow drugs to penetrate.
The problem with PDT techniques is that the light cannot penetrate very far into human tissue to activate the nanoparticles. Chen and his colleagues have discovered that Cu-Cy the nanoparticle's sensitivity to X-rays and the ability of X-rays to penetrate deeply into tissue means that the tumor can be deep inside tissue and still be effective. Another advantage of the Cu-Cy nanoparticle in combination with the X-rays is that no other photosensitizer needs to be used, making it convenient, efficient and cost-effective, according to the researchers.
Like some of the “theranostic” nanoparticles that are being developed, in which both therapeutic and diagnostic functions are combined into one nanoparticle, the Cu-Cy nanoparticle can serve both as treatment for cancer as well as for cell imaging.
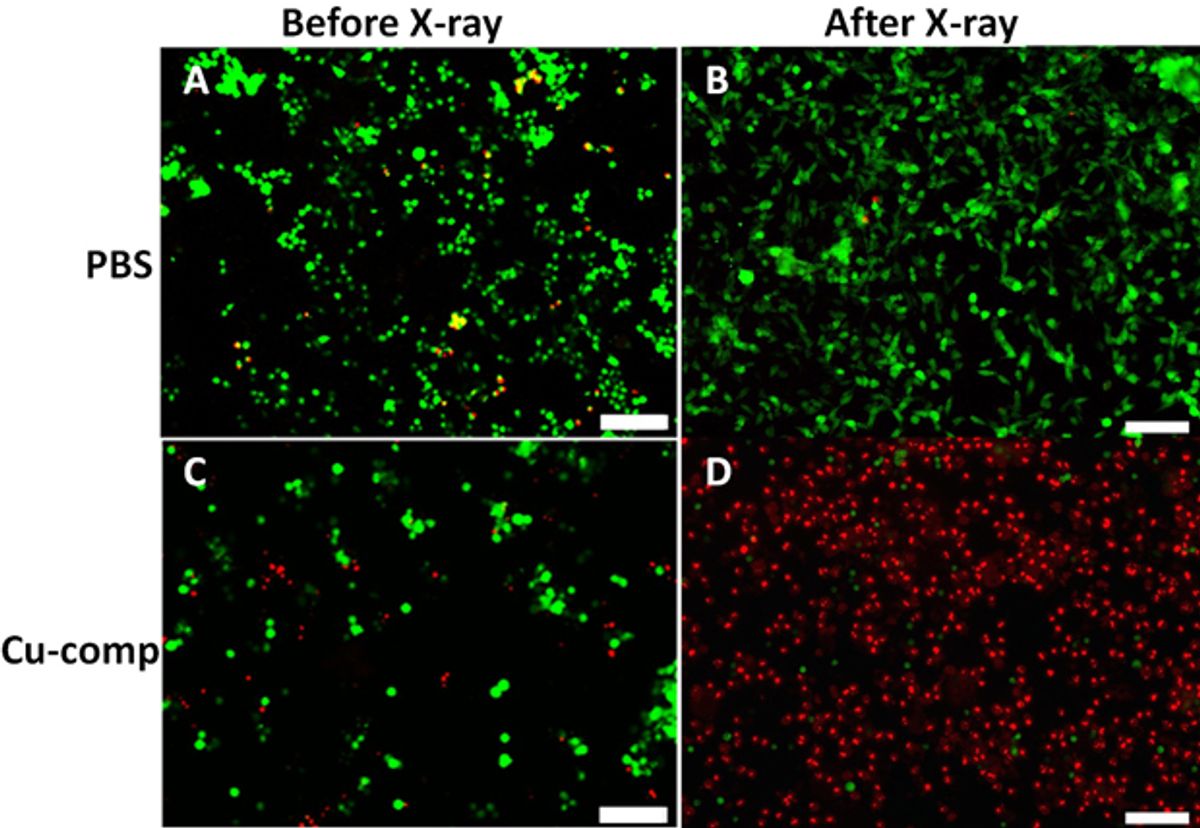
In experiments carried out thus far, Chen and his team have used the Cu-Cy nanoparticle and X-ray combination to treat a tumor. The results over a 13-day period showed that the tumor had not grown at all with the treatment while a tumor that had not been treated grew three times in size.
The UT Arlington has applied for a patent on the technology. Meanwhile Chen and his team are working on ways to shrink the size of the Cu-Cy nanoparticles, which currently are around 250 nanometers, so that the tumor tissue can more easily absorb them. If they can reduce the nanoparticles down to dimensions below 200nm, this should improve cell uptake. Ideally, they would like to bring those dimensions down to around 50 to 100nm.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.